
2. Solve the following expression for f:
m/c = w/f
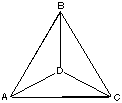
3. Point D lies in the center of the equilateral triangle ABC. What is angle ADB (in degrees)?
4. Multiply 4 x 1052 by 2 x 1060. Express your
answer in exponential form.
5.Given the following two equations with two unknowns, x and y. What is the value of x?
x + y = 1 3x + 5y = 4.2
6. The quadratic formula for the roots of x in a quadratic equation are
![]()
What are the values of x in the equation, x2 + 2 x - 3 =
0?
7. Use the quadratic formula in question 6 above, and determine the values of x in the following equation:
![]()
8. Use the quadratic formula in question 6 above, and determine the values of x in the following equation:
![]()
9. If 20.0 grams of sugar are dissolved in 80.0 grams of water, what
is the mass percentage of sugar in the solution?
10. How many grams of H2SO4 are contained in 125 mL of a solution that has a density is 1.80 g/mL and contains 96.0 mass % H2SO4?
11. Copper has a density of 8.92 g/cm3. If copper wire has
a diameter of 0.0500 cm, how many centimeters of wire must be measured
to obtain 2.00 g of copper?
12. How many square meters are there in an area of 72 square feet? (1
ft = 0.305 m)
13. The equation of a straight line is given by y = mx + b, where m is the slope and b is the intercept. What is the equation of the straight line below?

14. The graph below shows the vapor pressure of a certain liquid as a function of the temperature. Use the graph to answer the following questions:

This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem100/basicmath.html
Last revised: May 2 2001 by M. F. Richardson
© Brock University, 2001