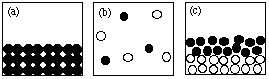
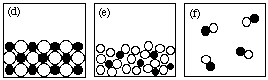
1. The figures shown below are particulate (submicroscopic) representations of various states of matter.


Answer the following questions by giving the letter of the figure that corresponds to the description. Drawings may be used more than once or not at all. There may be more than one answer to a given question.2. What is the formula of sulfuric acid?Write "NONE" if no drawing fits the description.
(i) Which drawing(s) represent submicroscopic particles in a sample of an element?
(ii) Which drawing(s) represent submicroscopic particles in a gas?
(iii) Which drawing(s) represent submicroscopic particles in a sample of a liquid compound?
(iv) Which drawing(s) represent submicroscopic particles in a sample of a molecular solid?
(v) Which drawing(s) represent submicroscopic particles in a sample of a heterogeneous mixture?
(vi) Which drawing(s) represent submicroscopic particles in a sample of an ionic solid?
(vii) Which drawing(s) represent submicroscopic particles in a sample of a compound?
(viii) Which drawing(s) represent submicroscopic particles in a gaseous mixture?
(ix) Which drawing(s) represent submicroscopic particles in a gaseous compound?
3. What is the formula for calcium chloride?
4. What is the formula for iron(III) oxide?
5. What is the systematic (IUPAC) name of P4O10?
6. What is the systematic (IUPAC) name of Cu(NO3)2?
7. Which of the following compounds is (are) ionic?
8. What is the oxidation number of phosphorus in H3PO3?
SO3 FeSO4 PCl5 HCl NaF
9. What is the oxidation number of nitrogen in Ca(NO2)2?
10. What is the oxidation number of sulfur in Na2S2O3?
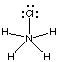
11. Which of the following Lewis (electron-dot) structures is/are wrong. (Give their letters.)
|
|
|
|
 |
 |
|
|
|
|
|
|
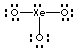
12. What do the letters VSEPR stand for?
13. Describe the geometric shapes of the molecules or ions whose Lewis structures are shown below. Your choices are
- linear
- bent
- triangular planar
- tetrahedral
- square planar
- triangular pyramid
- triangular bipyramid
- octahedral
- T-shape
|
|
 |
|
 |
|
|
|
|
|
|
|
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem100/compounds.html
Last revised: May 2 2001 by M. F. Richardson
© Brock University, 2001