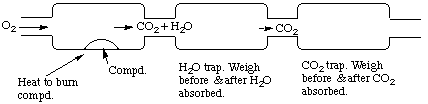
1. (27 marks)
(a) (3 marks)
(i) 1356.0(b) (3 marks)(ii) 445.7
(iii) 48.027
K2S(c) (4 marks)Al2O3
CaCl2
N2O3(d) (5 marks)NH4NO3
MnCO3
NaHSO3
A mole of phosphorus atoms contains 6.022 x 1023atoms.(e) (6 marks) Consider the ionA mole of P 4 molecules contains 6.022 x 1023molecules.
A mole of P 4 molecules contains 2.409 x 1024atoms.
A mole of phosphorus atoms has a mass of 30.974 grams.
A mole of P4 molecules has a mass of 123.90 grams.
The number of neutrons in this ion is 50The number of protons in this ion is 42.
The mass number is 92 .
The number of electrons is 40 .
The atomic weight of element X is 95.94.
The symbol for element X is Mo
(f) (6 marks) Balanced equations:
2 Fe + 3 Cl2 ---> 2 FeCl32. (18 marks)C6H6O + 7 O2 ---> 6 CO2 + 3 H2O
4 FeS + 7 O2 ---> 2 Fe2O3 + 4 SO2
(a) (5 marks) 3311 grams
(b) (4 marks)
1.00 kg/L(c) (3 marks) 40.0 g1.00 x 10-3 g/mm3
(d) (6 marks)
(i) 241.601 g/mol3. (10 marks)(ii) 59.6001 % O
38.77 % BaCl24. (12 marks)
(a) 213 g fluorine5. (15 marks) (a) Organic compounds burn in O2 to give CO2 and water, which can be trapped and weighed.(b) 303 g SF4
(c) 47.3% yield
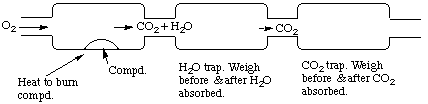
(b) C2H5O is the empirical formula
6. (6 marks)
(a) After reaction7. (12 marks; 2 marks each)
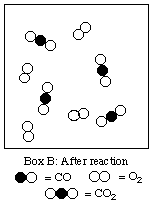
(b) 4
(c) O2 in excess by 4 molecules
(i) a(ii) c
(iii) b
(iv) d
(v) e
(vi) c
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Answers_Exam_1_1998.html
Created September 27, 2000 by M. F. Richardson
© Brock University, 2000