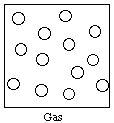
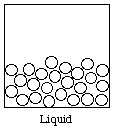
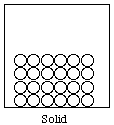
A. Particulare representations of gases, liquids, and solids.
Gas: No definite volume or shape; expands to fill the container; large spaces between particles (atoms or molecules)
Liquid: Definite volume, no definite shape; particles are close together but have no regular order
Solid: Definite volume, definite shape; highly regular arrangement of particles
If we let open circles represent the particles of matter, then the gas, liquid, and solid states can be represented as shown below.



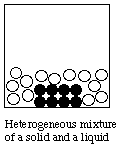
B. Particulate Representations of Homogeneous and heterogeneous mixtures
A homogeneous substance is completely uniform at the macroscopic level. It may be a single substance (e.g. water), or it may be a solution (e.g. air; a solution of salt in water).Below we use circles of different colors to represent different substances.A heterogeneous material is nonuniform at the macroscopic level: you can see that it consists of two or more substances. Examples are: a mixture of sand and sugar; a chocolate chip cookie.
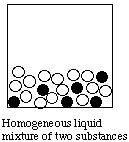

C. Particulate Representations of Molecules, Compounds, and Chemical Reactions
Two molecules of hydrogen react with one molecule of oxygen to form two molecules of water.
The hydrogen and oxygen molecules are diatomic. The water molecule is triatomic.
Hydrogen and oxygen are elements. Water is a compound of hydrogen and oxygen. A water molecule is always made up of two atoms of hydrogen for each atom of oxygen.
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_1.html
Created September 11, 2000 by M. F. Richardson
© Brock University, 2000