Lecture 11
Limiting Reagent and Percent Yield
A. A Simple Example of Limiting Reagent
In the last lecture, we made turkey sandwiches
according to the following "equation" (the recipe):
2 Bd + 3 Tk ---> Bd2Tk3
That is, one sandwich (Bd2Tk3) is made by
combining 3 slices of turkey with 2 slices of bread (Bd). Our
equation is balanced: the ingredients (reactants) are used up to form
the sandwich (product).
Now let's consider the concept of limiting reagent with
this same example. Suppose you have 10 slices of of bread and 24
slices of turkey. We can ask these questions:
- Which ingredient is used up first (the limiting reagent)?
- Which ingredient is left over?
- How much of it remains after the other ingredient is used up?
- How many sandwiches can you make?
Solution: Let's consider how much turkey we need to go with
10 slices of bread. Our "stoichiometric factor" from the above
equation is 3 slices Tk/2 slices Bd:
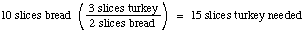
But we know that we have 24 slices of turkey, so we have more
turkey than we need to go with the 10 slices of bread. Thus the
bread is said to be the limiting reagent: it is used up first,
and limits the number of sandwiches that can be made by the recipe
given.
When we've made all the sandwiches we can, there will be 9
slices of turkey left over (24 available, 15 used in the
sandwiches.
If we use all our bread, we can make 5 sandwiches:
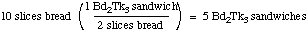
B. A Simple Example of % Yield
You used enough bread and turkey to make 5 sandwiches.
However, your dog grabs a sandwich while your back is turned. Now you
have only 4 sandwiches, not the 5 you could have made. So your %
yield is

C. A Chemical Example of Limiting Reagent: Production of
Ammonia, NH3
Ammonia is produced in a combination reaction between
hydrogen and nitrogen:
3 H2 + N2 ---> 2 NH3
Suppose we have 50.0 g H2 and 200. g N2.
Let's answer these questions, taking the molar masses of reactants
and products to 3 significant figures. (H2 2.02 g/mol;
N2 28.0 g/mol; NH3 17.0 g/mol).
- Which reactant is used up first (the limiting reagent)?
- Which reactant is left over?
- How much of it remains after the other reactant is used up?
- How much ammonia (in grams) can you make?
Solution: Step 1. First we need to determine the
moles of each reactant, since our balanced equation is in terms of
moles (3 moles of H2 react with 1 mole of N2 to
give 2 moles of NH3). We'll carry one extra significant
figure in our reported figures, and only round down at the end of the
calculations.
moles H2 = 50.0 g H2 x (1 mol
H2 / 2.02 g H2) = 24.75 mol H2
moles N2 = 200. g N2 x (1 mol
N2 / 28.0 g N2) = 7.143 mol N2
Step 2. Now let's calculate how much N2 is
needed to react with all the hydrogen:
(24.75 mol H2) x (1 mol N2/3 mol
H2) = 8.251 mol N2 needed
But we don't have 8.251 mol N2! We only have 7.143
moles, so N2 is limiting.
Step 3. If N2 is limiting, there must be leftover
H2. Let's check and see:
(7.143 mol N2) x (3 mol H2 / mol
N2) = 21.43 mol H2 needed to react with all the
N2. But we have 24.75 mol H2, so we have the
amount needed, plus some left over:
Have 24.75 mol H2
Use up 21.43 mol H2
Remaining: (24.75 - 21.43) mol H2 = 3.32 mol
H2 left over
Step 4. Since N2 is limiting, we have to use it
to calculate the amount of NH3 that can be made:
(7.143 mol N2) x (2 mol NH3 /
mol N2) x (17.0 g NH3 / mol NH3)
= 243 g NH3 is the maximum possible yield (all
N2 is used up)
D. A Chemical Example of % Yield: Production of Ammonia,
NH3
In the example in part C, suppose we actually obtain
211 g NH3. What is the % yield?

Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_11.html
Created October 3, 2000 by M. F. Richardson
© Brock University, 2000

