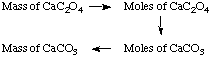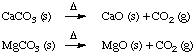Lecture 12
Chemical Analysis
A. Analysis of Mixtures When Only a Single Component Reacts
with the Reagent
Drug stores sell various mineral supplements. One of
these is a calcium-containing tablet, which contains powdered calcium
carbonate as the active ingredient. There is also an inert "binder"
that holds the calcium carbonate in its tablet shape. Thus the tablet
is a mixture of substances, only one of which is important in a
nutritional sense.
Let's suppose we want to analyze the tablet for its content of
CaCO3. We weigh the tablet, then add it to hydrochloric
acid so that the calcium carbonate will dissolve:
CaCO3 (s) + 2 HCl (aq) ---> CaCl2
(aq) + H2O (l) + CO2 (g)
We filter the solution to remove any insoluble material. Then, to
the filtered solution we add a solution of sodium oxalate in order to
precipitate the insoluble compound, calcium oxalate
(CaC2O4).
CaCl2 (aq) +
Na2C2O4 (aq) ---> 2 NaCl (aq) +
CaC2O4 (s)
We filter off the CaC2O4, dry it, and weigh
it.
Numerical Example. Suppose that a calcium-supplement tablet
weighs 1.250 g. After you carry out the above procedure, you obtain
1.508 g of dry CaC2O4. What percentage of the
tablet is CaCO3?
Molar masses: CaCO3 100.09 g/mol;
CaC2O4 128.10 g/mol
Solution. We will need to do mole/mass
conversions, and use the stoichiometric factors that relate moles of
calcium carbonate to moles of calcium oxalate, as depicted in the
following scheme:
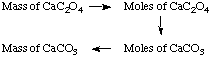
(1.508 g CaC2O4) x (128.10 g
CaC2O4/ mol CaC2O4) =
1.1772 x 10-2 mol CaC2O4
1.1772 x 10-2 mol CaC2O4 x (1 mol
CaCO3 / mol CaC2O4) = 1.1772 x
10-2 mol CaCO3
1.1772 x 10-2 mol CaCO3 x (100.09 g
CaCO3 / mol CaCO3) = 1.1783 g CaCO3
Thus the percent CaCO3 in the calcium tablet is
% CaCO3 = (1.1783 g CaCO3 /
1.250 g tablet) x 100
= 94.26% CaCO3
B. Analysis of Mixtures When Two Components React With the
Reagent
Numerical Example. The rock that Brock sits on
is dolomitic limestone, a mixture of both CaCO3 and
MgCO3. Both carbonates decompose on heating:
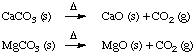
If 2.550 g of dolomite yields 1.341 g of MgO + CaO after heating,
what is the % CaCO3 in the dolomite? Report to 3 s.f.
Solution. Since we have two components, CaCO3
and MgCO3 , we might expect that we will have to use
algebra in order to solve this problem. So let's think about what we
know.
First, each mole of CaCO3 that decomposes gives a mole
of CO2; similarly, each mole of MgCO3 that
decomposes also gives a mole of CO2. Thus the total number
of moles of CO2 is equal to the moles of MgCO3
plus the moles of CaCO3:
Moles CO2 = moles MgCO3 + moles
CaCO3
The mass of CO2 released on heating is
2.550 g dolomite - 1.341 g of MgO + CaO = 1.209 g
CO2
We now find moles of CO2:
(1.209 g CO2 ) x (1 mol CO2 /
44.01 g CO2 ) = 0.27471 mol CO2 (1 extra s.f.)
Thus we now know that
0.27471 mol CO2 = moles MgCO3 +
moles CaCO3 (Eq. 1)
Now we have one equation but it contains two unknowns. So we have
to get a second relationship between our unknowns. We are told at the
beginning of this example that dolomite is a mixture of
CaCO3 and MgCO3.So we know that
mass dolomite = 2.550 g = mass MgCO3 + mass
CaCO3
Now we have another equation, also containing two unknowns. But we
know how to interconvert masses to moles, so let's do it. So let x =
mass CaCO3(in g). Then
2.550 g = x g + mass MgCO3
mass MgCO3 (in g) = 2.550 g - x
Now we get the moles of calcium and magnesium carbonates:
mol CaCO3 = (x g CaCO3) x (1 mol
CaCO3 / 100.09 g)
mol MgCO3 = (2.550 g - x g) x (1 mol MgCO3 /
84.31 g)
Substituting these into Eq. 1 above,
0.27471 = x / 100.09 + (2.550 - x) / 84.31 (Eq.
2)
Solving Eq. 2, we find that
x = 1.484 g CaCO3
And the percentage of CaCO3 is
% CaCO3 = (1.484 g CaCO3 / 2.550
g dolomite) x 100
= 58.2%
C. Combustion Analysis of Organic Compounds
Organic compounds containing only the elements C, H,
and O burn in air (or in O2) to give CO2 and
H2O as products. If the compound is burned in a
combustion apparatus (Fig. 4.12 in Kotz and Treichel),
CO2 and H2O can be separated from each other
and weighed. These data allow us to determine the empirical formula
of the compound.
Numerical Example. Suppose 1.042 g of an organic compound
containing C, H, and O is subjected to combustion analysis, and
yields 1.527 g CO2 and 0.6250 g H2O. What is
the empirical formula?
Solution: We will be able to get the masses of carbon and
of hydrogen from the masses of CO2 and H2O.
However, the compound also contains oxygen. In order to get the mass
of oxygen we will have to subtract the masses of carbon and hydrogen
from the starting mass of the compound (1.042 g).
moles CO2 = (1.527 g CO2 ) x (1
mol CO2/ 44.010 g CO2)
= 3.470 x 10-2 moles of CO2
mass of C = 3.470 x 10-2 moles of CO2 x (1
mol C / mol CO2) x (12.011 g C / mol C)
= 0.4168 g C
moles H2O = (0.6250 g H2O) x (1 mol
H2O / 18.015 g H2O)
= 3.469 x 10-2 moles of H2O
mass of H = 3.469 x 10-2 mol H2O x (2 mol H
/ mol H2O) x 1.0079 g H / mol H)
= 0.06993 g H
mass of O = 1.042 g of compound - mass C - mass O
= 1.042 g - 0.06993 g - 0.4168 g
= 0.5553 g O
Now that we have the masses of C, H, and O, we can determine the
moles, and then the mole ratio that gives us the empirical formula.
moles C = (0.4168 g C) x (1 mol C / 12.011 g C) =
0.03470 mol C
moles H = (0.06993 g H) x (1 mol H / 1.0079 g H) = 0.06938 mol H
moles O = (0.5553 g O) x (1 mol O / 15.999 g O) = 0.03471 mol O
Divide by moles of C to get the mole ratios of H to C and O to C:
0.06938 mol H / 0.03470 mol C = 1.999 mol H / mol C
0.03471 mol O / 0.03470 mol C = 1.000 mol O / mol C
Hence, since there are two hydrogens and one oxygen for every
carbon, the empirical formula is CH2O.
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_12.html
Created October 5, 2000 by M. F. Richardson
© Brock University, 2000