A. Overview: a simple reaction
Let's imagine we are in the lab. We take a piece of metallic zinc and drop it into a beaker that contains dilute hydrochloric acid (HCl). Soon we notice two things:
- Bubbles begin to appear in the solution and rise to the surface
- The piece of zinc becomes smaller
We could analyze the gas; if we did, we would find that it is the gas hydrogen, which is made up of H2 molecules. We could also analyze the solution and determine that it now contains zinc chloride, ZnCl2. We could even determine that every mole of zinc that reacts produces a mole of hydrogen gas and a mole of zinc chloride.
The balanced equation for this reaction is
Zn (s) + 2 HCl (aq) ---> ZnCl2 (aq) + H2 (g) This reaction is an example of two different classes of chemical reactions:
- It is a gas-forming reaction: the gas H2 is produced. (Lecture 14)
- It is a redox reaction: zinc metal is oxidized to zinc(II) ion, while hydrogen ions are reduced to gaseous elemental hydrogen. (Lecture 15)
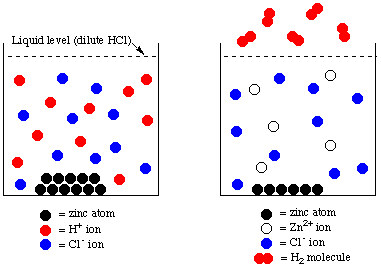
B. Particulate view of the reaction of zinc with hydrochloric acid: spectator ions and the net ionic equation

On the left above we have a particulate view of the reaction mixture just at the point the zinc metal has been dropped into the HCl solution. HCl is a strong electrolyte: it dissociates completely into the ions H+ and Cl- when it dissolves in water. The red circles represent H+ ions and the blue ones are Cl- ions. You can see that there are 10 red circles and 10 blue ones, representing the equal numbers of H+ and Cl- ions.
The black circles represent the piece of solid zinc. Water molecules aren't shown, but they fill the spaces between the H+ and Cl- ions.
Now look at the right hand part of the figure. The reaction has gone as far as it can: all the hydrogen ions (10 of them) have been converted to H2 molecules. Five atoms of zinc have become Zn(II) ions, in accordance with the balanced equations. All the chloride ions remain in solution, unchanged during the reaction. The chloride ions are called spectator ions they just sit and watch the action taking place between zinc metal and the hydrogen ions.
The net ionic equation is the balanced equation that shows only the species that undergo chemical change. In this instance, the species undergoing change are zinc metal and hydrogen ions. Thus the net ionic equation for the reaction between zinc metal and aqueous hydrochloric acid is
Zn (s) + 2 H+ (aq) ---> Zn2+(aq) + H2 (g) Net ionic equations are discussed in more detail in Kotz and Treichel section 5.6, and will be discussed further in this lecture (Section E).
C. Strong electrolytes, weak electrolytes, nonelectrolytes
Electrical conduction is due to the movement of charged particles. These can be electrons (as in conduction through a wire), or ions (as in conduction through an aqueous solution or a molten salt).
Compounds can be divided into 3 classes according to the ability of their aqueous solutions to conduct electricity. These are:
- strong electrolytes: produce highly conducting solutions because they dissociate completely into their ions in water.
- weak electrolytes: produce poorly conducting solutions because they dissociate only slightly in water.
- nonelectrolytes: aqueous solutions do not conduct electricity because nonelectrolytes do not dissociate into ions when they dissolve in water.
Among the strong electrolytes are: all salts; strong acids (HCl, HNO3, H2SO4, and a few others; see Table 5.2 in your text); and strong bases (LiOH, NaOH, KOH, Ba(OH)2, etc.)
The weak electrolytes include: weak acids (organic acids such as acetic acid; H3PO4, H2CO3, and other inorganic acids except the strong ones); weak bases (NH3 and organic amines)
Nonelectrolytes are nonionic molecular compounds that are neither acids nor bases. Sugar, ethyl alcohol, and millions of other organic compounds fall into this category. Some inorganic compounds are also nonelectrolytes.
D. Precipitation reactions: the solubilities of salts
A precipitation reaction is said to occur when the mixing of two homogeneous solutions results in the formation of a solid. An example is the reaction of aqueous sodium chloride and silver nitrate solutions: the silver ions and chloride ions combine to produce the white, insoluble salt AgCl, while the sodium and nitrate ions remain in solution. The balanced equation is
NaCl (aq) + AgNO3 (aq) ---> AgCl (s) + NaNO3 (aq) The particulate view shows clearly that the sodium and nitrate ions are spectator ions:
Thus the net ionic equation for this reaction is:
Ag+ (aq) + Cl- (aq) ---> AgCl (s) Salt solubilities. A table for solubilities of salts is shown in Kotz and Treichel, Figure 5.4.
E. Some examples of precipitation reactions; net ionic equations
Example 1. What will happen if we mix aqueous solutions of BaCl2 and Na2SO4? Write the overall and the net ionic equations if there is a reaction.
Solution: We see from Figure 5.4 in Kotz and Treichel that all sulfates are soluble except for the sulfates of Sr2+, Ba2+, and Pb2+. All chlorides are soluble except for those of Ag+, Hg22+, and Pb2+. Thus we expect BaSO4 to precipitate from solution.Overall equation: BaCl2 (aq) + Na2SO4 (aq) ---> BaSO4 (s) + 2 NaCl (aq) To get the net ionic equation, the easiest way is to start with the overall equation. Let all the soluble salts or other strong electrolytes dissociate into their ions. Leave weak electrolytes, nonelectrolytes, or insoluble species in their molecular form. Thus for the above equation,
Ba2+ (aq) + 2 Cl- (aq) + 2 Na+ (aq) + SO42- (aq) ---> BaSO4 (s) + 2 Na+ (aq) + 2 Cl- (aq)
Now cancel like ions on each side (2 Cl- and 2 Na+) to obtain the net ionic equation:
Net ionic equation: Ba2+ (aq) + SO42- (aq) ---> BaSO4 (s) Example 2. What will happen if we mix solutions of Fe2(SO4)3 and NaCl?
Solution. From the solubility rules given in example 1 above, we predict that there will be no reaction. Hence there are no equations to write.
Example 3. What will happen if we mix aqueous solutions of Na3PO4 and Ca(NO3)2?
Solution. All nitrates are soluble, but all phosphates are insoluble except for those of NH4+ and the alkali metal cations. Thus we expect to have a precipitate of Ca3(PO4)2.The equations are:
Overall: 2 Na3PO4 (aq) + 3 Ca(NO3)2 (aq) ---> Ca3(PO4)2 (s) + 6 NaNO3 (aq) Net ionic: 3 Ca2+ (aq) + 2 PO43- (aq) ---> Ca3(PO4)2 (s)
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_13.html
Created October 10, 2000 by M. F. Richardson
© Brock University, 2000