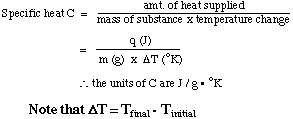
A. Overview: 2 Key Questions About a Chemical Reaction
1. Will the reaction "go" to form the desired product? This question can be answered by thermodynamics. (Chapters 6 and 20, Kotz & Treichel, 4th ed.) We will begin our study of thermodynamics with today's lecture.
2. How fast will the reaction "go?" This question can be answered by kinetics, which is the study of reaction rates. (Chapter 15, Kotz and Treichel).
B. Reactions That "Go"
If you hold a flame to a piece of paper, the paper catches fire and burns to produce carbon dioxide and water. Paper belongs to the class of compounds called carbohydrates, whose general formula is Cn(H2O)m. The reaction that takes place when the paper burns is
Cn(H2O)m (s) + O2 (g) ---> n CO2 (g) + m H2O (l)
reaction is product-favoredThe burning of paper is said to be product-favored because the reactants combine to produce significant amounts of the products.
This reaction takes place extremely slowly at room temperature, but very rapidly at high temperatures. We will learn why this is so when we study kinetics next term.
Note that the reverse reaction, the combination of carbon dioxide with water to produce carbohydrates and oxygen, does not "go" to any appreciable extent: the reverse reaction is reactant-favored.
n CO2 (g) + m H2O (l) ---> Cn(H2O)m (s) + O2 (g)
reaction is reactant-favoredIf we want to make carbon dioxide combine with water to produce carbohydrates and oxygen, we have to supply energy. This is not easy to do in a chemistry laboratory, but nature does it by the process called photosynthesis. Plants capture light energy from the sun and use it to convert carbon dioxide and water to biomass (carbohydrates) and oxygen.
Since energy transfer accompanies all chemical reactions, it is important to understand energy in order to know why reactions occur as they do, and to be able to predict whether reactions will occur or not.
C. Forms of Energy
Among the forms of energy are kinetic energy and potential energy. Kinetic energy is the energy of motion and includes these forms:
- mechanical energy or work
- thermal energy (heat)
- electrical energy
- sound
Mechanical energy involves the motion of a macroscopic object across a distance, e.g. a moving automobile. The other three forms of energy listed above involve the motion of submicroscopic particles: heat is due to the vibration of molecules; electrical energy is due to the movement of electrons through a wire; and sound is due to the compression and relaxation of the spaces between molecules.
Potential energy results from an object's position. Examples are:
- gravitational energy (energy of a rock on top of a cliff)
- chemical energy (energy in the bonds between atoms)
- electrostatic energy (separation of positive and negative charges)
All the forms of energy can be interconverted. Chemical energy becomes thermal energy when paper burns; electrical energy becomes mechanical energy when a fan is turned on; electrostatic energy becomes light and sound when lightning appears in the sky.
The Law of Conservation of Energy: Energy is neither created nor destroyed, but can be converted from one form to another.
D. Units of Energy
The amount of heat (energy) contained by an object must be distinguished from the temperature of that object. For example, a burning piece of paper and a house on fire might both have the same temperature, but the amount of heat generated by each is quite different. In other words, the heat or energy content is related to the mass of the object.
Two forms of energy that are especially important are heat and work. Heat is measured in terms of calories (cal):
One calorie is the amount of heat needed to raise the temperature of one gram of pure water from 14.5 to 15.5o C. One kilocalorie (kcal) is 1000 calories.
In food chemistry, one Nutritional calorie is actually equal to 1000 calories. One nutritional calorie is abbreviated 1 Cal. Note the difference between 1 cal (not capitalized) and 1 Cal (capitalized!)
Work is measured in Joules (J). One Joule is the work required to accelerate a mass of 1 kg by 1 m / s2 over a distance of one meter.
Work (w) is defined as the amount of energy associated with applying a force (F) over a given distance (d), or
w = F x d
Now since force is equal to the mass of the object multiplied by the acceleration (F = m x a), we have
w = (m) x (a) x (d)
= (1 kg) x (1 m / s2) x (1 m)
= 1 kg.m2 / s2
= 1 J
Work and heat are interconvertible forms of energy. The relationship between joules and calories is
4.184 J = 1 cal
E. Heat Capacity and Specific Heat
The heat capacity of an object is the amount of heat necessary to raise the temperature of the object by exactly 1oC. The greater the mass of the object, the greater the amount of heat it will take to increase its temperature by a certain amount. Thus the heat capacity is an extensive property of the substance (See Chapter 1.4 in Kotz and Treichel).
The specific heat capacity (usually called the specific heat, and abbreviated C) is an intensive property of that substance: it is the heat required to raise the temperature of one gram of the substance by 1oK. The units of the specific heat are J / g.oK, as shown below.
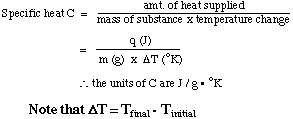
If the temperature of the substance increases, then the temperature change is positive and the substance absorbs heat. If the temperature of the substance decreases, then the temperature change is negative and the substance loses heat.
Determining specific heat: an example: The specific heats of solids are readily determined if they do not react with or dissolve in water. Suppose that a 25.64 g sample of unknown metal is heated to 100.00 oC in boiling water. When it is added to 50.00 g of water in an insulated container, the water temperature increased from 25.10 to 28.49 oC. What is the specific heat of the metal in J/g.oK?
Analysis. The metal loses heat (qmetal is negative) when it is put into the insulated container, and the water in the container gains heat qwater is positive). The law of conservation of energy requires that
qmetal + qwater = 0 or, qwater = - qmetal This problem is easiest to solve if you make a table that contains all the data you know about the system. Remember that the specific heat of water, 1 cal/g.oK, corresponds to 4.184 J/g.oK since one calorie is equal to 4.184 Joules. Also note that the temperature difference in oC is the same as the temperature difference in oK: one Celsius degree has the same magnitude as one Kelvin degree.
|
|
|
Sp. heat, J/g.oK |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Answer. The specific heat of the metal is 0.387 J/g.oK.
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_18.html
Created October 22, 2000 by M. F. Richardson
© Brock University, 2000