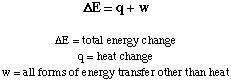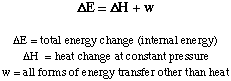Lecture 19
Enthalpy, Heat, and Work
A. Heats associated with phase changes
If ice is heated, it becomes liquid water as it
absorbs heat. As more heat is absorbed, it eventually boils and
becomes gaseous water or steam. Water is said to undergo
phase changes during this heating process. A phase is a
physically distinguishable state of matter.
There are six possible phase changes for any given chemical
substance, and each has a specific name. These are
- solid to liquid: fusion (melting)
- liquid to solid: crystallization (freezing)
- liquid to gas: vaporization (boiling)
- gas to liquid: condensation
- solid to gas: sublimation
- gas to solid: deposition
These phase changes are summarized in the figure below.
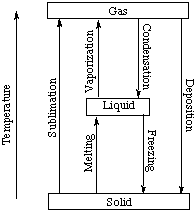
Each phase change requires or releases heat, depending on the
direction of the change. Let's consider the heat changes for some of
these processes, with water as our example.
- The heat of fusion is defined as the quantity of heat
needed to melt one gram of ice at 0oC to produce one
gram of liquid water at 0oC. The amount of heat
required for this process is 333 J/g. The molar heat of
fusion for ice is (333 J/g)(18.01 g/mol) = 6.00 kJ/mol
- The heat of crystallization is the quantity of heat
released when one gram of water at 0oC freezes to ice
at 0oC. The amount of heat released is the negative of
the heat of fusion, or -333 J/g.
- The heat of vaporization of water is the quantity of
heat needed to vaporize one gram of liquid water at 100
oC to produce one gram of steam at 100 oC.
The amount of heat required for this process is 2260 J/g, much
greater than the heat of fusion!
- The heat of condensation of water is the heat released
when one gram of steam at 100 oC condenses to one gram
of liquid water at oC. It is equal to -2260 J/g. This
means that when steam condenses, the energy is lost by the steam
and gained by the surroundings.
B. What happens to the temperature during the process, solid
--> liquid --> gas?
Let's take ice as an example. If we have ice at a
temperature below 0 oC, the application of heat first
warms the ice to its melting point, 0 oC. While the ice is
melting, all the heat absorbed goes to melt the ice and the
temperature doesn't change.
When the ice has melted, further absorption of heat increases the
temperature of the liquid water until the boiling point is reached.
When water begins to boil, once again the temperature remains fixed
until all the water has vaporized to gaseous water vapor (steam).
Only after all the liquid has vaporized does the temperature increase
again. This process is shown graphically below for water:
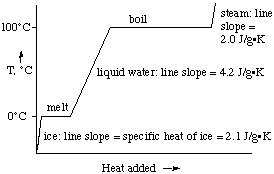
Each chemical substance has characteristic values for the specific
heats of each phase, and for the heats of the phase changes, but the
graphs of temperature vs. heat added are similar to the one above for
water.
C. The Relation Between Total Energy, Heat, and Work
System: that portion of the universe of interest to
the experimenter. Surroundings: everything else!
If I drop my pen, the pen is the system. Everything else (the
floor) is the surroundings. the potential energy of the pen while I
held it up was transferred to the floor as heat when the pen hit the
floor. The system (the pen) lost energy when it fell to the floor;
the surroundings (the floor) gained energy.
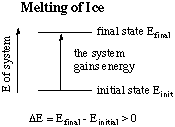
For a chemical example, consider what happens as ice melts. The
system (ice) gains energy, while the surroundings lose energy.
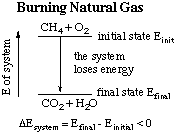
As a second example, consider how many Canadians heat their homes
in winter: by burning natural gas (methane, CH4). Here,
the initial state of our system is methane and oxygen; the final
state is carbon dioxide and water. Energy is lost by the system, but
gained by the surroundings (the homes).
So far we have considered only changes in the heat energy.
However, the total energy change is equal to the heat change
plus any work done. This is a statement of the Law of Conservation
of Energy, also known as The First Law of Thermodynamics.
Mathematically this law is expressed as
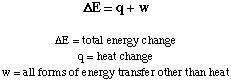
The numerical values of q and w have a positive or negative sign
depending on the system's perspective:
- If the system loses energy (as heat), q is negative
- If the system gains heat, q is positive
- If the system loses energy by doing work, w is negative
- If the system gains energy by having work done on it, w is
positive
D. Two Important Experimental Conditions: Constant Pressure,
Constant Volume
Experiments are usually carried out either at constant
pressure (as you do in the lab when you mix two solutions in a beaker
or flask), or at constant volume (as you might if you allowed dry ice
to sublime in a sealed steel container).
If the experiment is carried out at constant pressure and there is
a volume change, then work is done, either by the system or on it.
This is called pressure-volume work. If we think about dry
ice subliming in a balloon, the balloon expands against atmospheric
pressure. Now pressure is defined as the force applied over a unit
area (P = force/area), and the volume is equal to the area multiplied
by the height (V = area x height). Thus the work done is
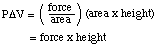
The units of PV are the same as the units of work: work is defined
as application of a force over a distance. This is why we refer to
pressure-volume work for a process that occurs with a volume
change at constant pressure.
If the experiment is carried out at constant volume, then no work
is done either by the system or on it.
The heat transferred in or out of a system at constant pressure is
the enthalpy change. The abbreviation for enthalpy is
H. Thus we have
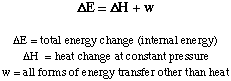
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_19.html
Created October 24, 2000 by M. F. Richardson
© Brock University, 2000