Dalton's theory arose from Lavoisier's Law of Conservation of Matter, and work by Proust that showed that different samples of the same compound had a fixed composition (Law of Constant Composition). On the basis of these two laws, Dalton proposed the following:
1. All matter is made of atoms. Atoms are indivisible and indestructible.
2. All atoms of a given element are identical. Atoms of different elements have different masses and different properties.
3. Compounds are formed by a combination of two or more different kinds of atoms. Atoms combine in the ratio of small whole numbers.
4. Atoms are the units of chemical change. Reaction involves only combination, separation, or rearrangement of atoms, but atoms are not created, destroyed, divided, or converted into other kinds of atoms during a chemical reaction.
Dalton's data allowed him to make the following statement, called Dalton's Law of Multiple Proportions. If 2 elements form two different compounds, the mass ratio of the elements making up one compound is a whole number multiple of the mass ratio of the elements in the second compound.
Example: CO, CO2
CO is made of C and O in the proportion 16 g O/12 g C = 1.333
CO2 is made of C and O in the proportion 32 g O/12 g C = 2.6672.667/1.333 = 2
Modern Statement of the Law of Multiple Proportions: We now have analyses on many more compounds than were available to Dalton, and the Law of Multiple Proportions must be modified slightly. The modern version is this: If two elements form two different compounds, the mass ratio of the elements making up the first compound and the mass ratio of the elements making up the second compound are in the ratio of small whole numbers.
Example: CrO2, Cr2O3
CrO2 is made up of Cr and O in the proportion 32 g O/52 g Cr = 1.625
Cr2O3 is made up of Cr and O in the proportion 48 g O/52 g Cr = 2.1672.167/1.625 = 1.333 = 4:3
Electricity - now known to be due to the electron. Electricity is the flow of electrons.
Radioactivity
1896: Becquerel discovers that uranium ore emits rays that expose a photographic plate
1898: Marie Curie proposes that these rays come from disintegrating atoms
Radioactive elements emit 3 kinds of radiation: a (alpha), b (beta), and g (gamma). Fig. 2.2 in Kotz and Treichel shows that alpha particles are attracted to the negatively charged plate, beta particles are attracted to the positively charge plate, and gamma rays do not deviate from a straight line. Thus,
- alpha particles are positively charged (they are helium nuclei)
- beta particles are negatively charged (they are electrons)
- gammas have no charge at all (they are pure energy)
Faraday and Electrolysis
Faraday showed that passing an electric current through a solution of certain metal salts (such as tin chloride; Fig. 2.3 in Kotz and Treichel) causes the salt to decompose into the elements. He determined that the same amount of electricity could produce different quantities of different metals, and postulated that these masses were related to the relative masses of the atoms of different elements.
Just as the atom is the fundamental particle in chemistry, Faraday's experiments were interpreted to mean that there was a fundamental particle of electricity: the electron.
Electrons: mass and charge
Fig. 2.4 in Kotz and Treichel: in a cathode ray tube, beta particles are attracted to the positive electrode (top) and are deflected by a magnetic field (bottom). The deflection in the magnetic field is related to the magnitude of the field, and the mass and velocity of the particles. These experiments show that the electron is negatively charged.
Fig. 2.5 in Kotz and Treichel: Thomson applied magnetic and electrical fields simultaneously to a beam of electrons (beta particles). The electrical field deflects the electrons in one direction, and the magnetic field in the other. By simultaneously adjusting the fields until there was NO deflection, Thomson was able to determine the ratio of the mass to the charge of an electron.
Fig. 2.6 in Kotz and Treichel: Millikan determines the exact charge of an electron. Oil droplets are dispersed in a chamber that contains some air. There is a source of x-rays that cause the air molecules to ionize and produce electrons, e.g.
O2 ---> O2+ + e-
When an electron hits an oil droplet, it can stick so that the oil drop now has a negative charge. Some oil drops have one electron, some have more than one, and some will have none. The oil drops tend to settle by the force of gravity; however, the charge can be adjusted on the electrical plates so that a particular oil drop doesn't move (it is equally attracted downward by gravity and upward by the positive charge on the upper plate). Knowledge of the size of the oil drop, the force of gravity, and the charge on the electrical plates allows accurate calculation of the charge on the electron.
Protons
Fig. 2.7 in Kotz and Treichel: application of high voltage to a cathode ray tube containing a gas not only generates "cathode rays" (negatively charged particles - electrons - that are attracted to the positively charged cathode), but also generates "canal rays" that move in the opposite direction, towards the negatively charged electrode or anode. Different gases produce positively charged particles with different mass/charge ratios.
The use of hydrogen gas gives positively charged particles with the lowest mass/charge ratio, suggesting that hydrogen provides the smallest particle that has a positive charge. This particle is the proton.
Neutrons
Postulated to exist because the atomic weights of atoms were greater than could be accounted for by the protons and electrons; experimentally observed by Chadwick in 1932.
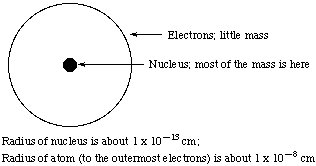
Rutherford designed an experiment to investigate atomic structure in which he bombarded gold foil with alpha particles, which are positively-charged helium nuclei. (See Fig. 2.8 in Kotz and Treichel.) Most of the alpha particles shot straight through the gold foil as though there was nothing to stop them! A few were deflected, some by angles of 180 degrees, which means that the alpha particle bounced right back in the direction from which it had come.
These results could only be interpreted to mean that the nuclei, which contain most of the mass of the atom, are in fact very very tiny. So it is very unlikely that a tiny alpha-particle will collide with a tiny gold nucleus. If it doesn't collide with a gold nucleus, it passes straight through the gold foil. Only if it happens to hit a gold nucleus will it be deflected from its straight-line path; a "head-on" collision is necessary for the alpha particle to bounce back in the direction from which it came. (Fig. 2.9, Kotz and Treichel)
Note that if an alpha particle hits an electron, not much will happen to the alpha particle because it has about 10,000 times the mass of an electron!

This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_4.html
Last modified September 20, 2000 by M. F. Richardson
© Brock University, 2000