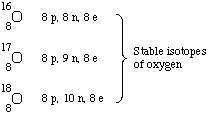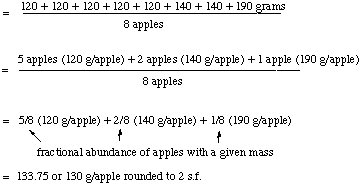Lecture 5
Atoms, Isotopes, Atomic Weights
A. Atomic Composition; Isotopes
The nucleus is made up of protons and neutrons. The
protons determine the chemical identity of the element (because the
number of electrons in a neutral atom is equal to the number of
protons, and it's the electrons that are responsible for the
chemistry of an element).
The atomic number of an atom is the number of protons in
its nucleus, and is written as a left subscript. The mass number is
the number of protons plus the number of neutrons, and is written as
a left superscript. The element symbol can be obtained from the
atomic number or vice versa from the periodic table.
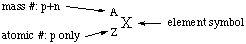
Isotopes are atoms which have the same atomic number but
different mass numbers. Thus they are atoms of the same element whose
masses are different because the number of neutrons is different.
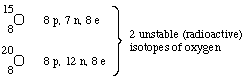
Some isotopes are radioactive, some are not. For example, oxygen has
3 naturally occurring, nonradioactive isotopes and several
radioactive isotopes that are not found naturally.
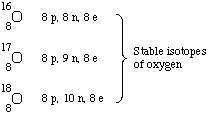
B. The Mass Spectrometer
In a mass spectrometer (Fig. 2.11, Kotz and
Treichel), a gaseous sample is introduced into an ionization chamber.
An electric discharge (the "electron gun") bombards the sample with
electrons and causes ionization to occur. Positive ions are
accelerated towards a detector, but their path is controlled by a
magnetic field that allows only ions of a particular mass to reach
the detector at a given time. Variation of the magnetic field results
in all ions reaching the detector but at different times, thus
resulting in a mass spectrum in which ions of different
masses appear at different places on the chart.
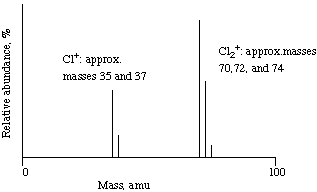
Below is a schematic mass spectrum for chlorine gas. The electrons
from the electron gun strike the Cl2 molecules, resulting
in two processes occurring:
Process 1: An electron is knocked off of a
Cl2 molecule, resulting in Cl2+
ions:
Cl2 + e --->
Cl2+ + 2 e
Process 2: The incoming electron breaks the Cl2
molecule apart:
Cl2 + e ---> Cl +
Cl+ + 2 e
The positive ions (Cl2+ and Cl+)
are accelerated towards the detector, where their relative abundances
are recorded. The mass spectrum shows two clusters of peaks: one
cluster consists of two peaks, at masses of about 35 and 37, and the
other cluster has three peaks, at masses of 70, 72, and 74.
The appearance of two peaks at masses 35 and 37 shows that
chlorine has two stable isotopes. The three peaks at masses 70, 72,
and 74 are due to the combinations of two 35Cl atoms, one
35Cl and one 37Cl, and two 37Cl
atoms, respectively.
C. Exact Determination of Atomic Masses from the Mass Spectrum
Very accurate measurements can be made by means of a
mass spectrum. The peak cluster that is due to Cl+ can be
analyzed as follows:
Peak at mass 35 (approx.): exact mass is 34.96885 amu;
75.77% of the total Cl+ cluster.
Peak at mass 35 (approx.): exact mass is 36.96590 amu; 24.23% of
the total Cl+ cluster.
(1 amu = 1/12 of the mass of an atom of 12C)
The atomic weight of chlorine is the average mass of a chlorine
atom. We will calculate the atomic weight for chlorine in Section E
below.
D. Calculation of Average Mass
Before we calculate the atomic weight of chlorine,
let's consider a simpler example. Suppose I buy a basket of 8 apples.
I weigh each one and find the following results
- 5 apples each have a mass of 120 g
- 2 apples each have a mass of 140 g
- 1 apple weighs 190 g
Now to get the average mass, I add up all the individual masses
and divide by the number of apples. But let's show how this relates
to fractional or percentage abundances, so we have a
closer relationship to the data from the mass spectrum of chlorine.
Thus,
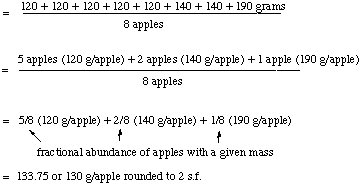
Note that we have derived a very simple way to calculate the
average mass when we know the percentage or fractional abundance of
each object with a given mass. (Remember that % = fractional
abundance * 100).
Average mass = (fraction of A)*(mass of A) + (fraction of
B)*(mass of B) + ...
E. The Atomic Weight of an Element
Now we know the atomic masses for atoms of each of the
two chlorine isotopes. The atomic weight of an element is the
average mass of an atom of that element. The average is taken
over a large number of atoms: each peak in a mass spectrum is due to
millions of ions.
Let's use the data in Section C above to calculate the atomic
weight of chlorine.
75.77 % 35Cl = 0.7577 fractional
abundance
24.23% 37Cl = 0.2423 fractional abundance
Thus, since the average mass of a chlorine atom equals the atomic
weight of chlorine,
Atomic weight of chlorine = 0.7577 * 34.96885 amu/atom
+ 0.2423 * 36.96590 amu/atom
= 35.45 amu/atom
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_5.html
Last modified September 20, 2000 by M. F. Richardson
© Brock University, 2000