
|
|

|

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notice two things from these data:
1. Similarity of the formulas of some of the oxides:
Li2O and Na2O
BeO and MgO
B2O3 and Al2O32. Trend in densities: trend in densities from Na through Al is similar to the trend for Li through C
Now, in order to construct Mendeleev's periodic table, what we do is the following:
So let's do this for the first 15 elements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
So far, so good. But let's look at what Mendeleev did next, as he continued to add elements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Why did Mendeleev put three elements into "Gruppe VIII?' Why not have 10 groups? Partly because the European musical scale is based on the octave (8 notes), which was deemed "perfect" and a gift from God. Thus, eight groups of elements was thought to be divinely ordained.
When the rare gases (He, Ne, Ar, etc.) were discovered and their atomic weights were determined, anomalies began to arise when the elements were arranged according to atomic weight. The atomic weight of Ar is greater than that of K so K now appears in Gruppe VIII, and Ar appears in Gruppe I.
Cl: atomic weight 35.4
K: atomic weight 39.1
Ar: atomic weight 39.9
Ca: atomic weight 40.1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The chemistry of Ar is unlike the chemistry of Li , Na, and Cu; in fact the chemistry of Cu doesn't much resemble the chemistry of Na and Li. So the idea of placing the elements so that a column would include elements with similar chemistry begins to fall apart. "Gruppe 8" is even worse: it contains two extremely unreactive gases (He and Ne), one violently reactive metal (K), and 3 moderately reactive metals (Fe, Co, and Ni).
Mendeleev did his work before much was known about atomic structure. Once protons and electrons had been discovered, the way was opened to relate periodicity to atomic number rather than to atomic weight. Why atomic number? Because the atomic number is equal to the number of electrons in a neutral atom, and it is the electrons that determine chemical behaviour.
Let's look at the elements H, Li, Na, and K, which are in "Gruppe I" in Mendeleev's periodic table. They have the following numbers of electrons in their shells:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
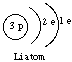
Or, in pictorial form, the atomic shell structures for H, Li, Na, and K look like this:

|

|

|

|
The outermost shells of H, Li, Na, and K each contain a single electron: This is the reason for the similarities between these elements.
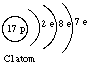
Note that the Mendeleev Group Number (I for H, Li, Na, and K) corresponds to the number of electrons in the outermost shell of the atom. This is generally true. For example, shell structures are shown below for an oxygen atom (Group VI) and a chlorine atom (Group VII).

|
|
The concept of electron shells, and the fact that the number of electrons is equal to the number of protons in the nucleus (the atomic number), led to the following idea:
Arrange elements according to atomic number rather than according to the atomic weight.
Thus the modern periodic table was born.
Here are the first 36 elements, arranged by atomic number so that they fall into groups (columns) of elements whose properties are similar. Similarities continue beyond the rows shown here.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
He |
|
Li |
Be |
|
|
|
|
|
|
|
|
|
|
B |
C |
N |
O |
F |
Ne |
|
Na |
K |
|
|
|
|
|
|
|
|
|
|
Al |
Si |
P |
S |
Cl |
Ar |
|
K |
Ca |
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
Ga |
Ge |
As |
Se |
Br |
Kr |
There are several differences between this periodic table and Mendeleev's periodic table.
We will explore the ideas of electron shells and the electronic structure of the atom in more detail later this term.
D. Properties and Chemistry of Some Elements in the Periodic Table
Read Section 2.8 in the 4th edition of Kotz and Treichel.
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_6.html
Last modified September 22, 2000 by M. F. Richardson
© Brock University, 2000