Lecture 8
Atoms, Molecules, Moles, Molar Mass
A. The Mole Defined
In everyday life, we use terms like "pair," "dozen,"
or "ream" to refer to a specific number of items (a ream is 500).
In Chemistry, we could always just specify the number of atoms or
molecules that are needed for some purpose. This would be cumbersome,
because atoms and molecules are so small that the numbers needed are
unimaginably huge. So we need to define a unit that is convenient for
specifying a sensible number of atoms or molecules. That unit is
the mole.
The Mole: Definition. The mole is defined as the mass of a
substance that contains the same number of particles as there are
atoms in exactly 12 grams of carbon-12.
Measurements show that there are exactly 6.022 x 1023
carbon atoms in 12 grams of carbon-12. Thus one mole of carbon-12 has
a mass of exactly 12 g, as this is the mass that contains 6.022 x
1023 carbon-12 atoms.
Just as the number "12" is called a dozen, the number 6.022 x
1023 is called Avogadro's number.
B. Application of the Mole to Chemical Reactions
Consider the reaction between sodium and chlorine to
produce sodium chloride,
2 Na + Cl2 ---> 2 NaCl.
The equation tells us that two atoms of sodium react with one
molecule of chlorine to give two formula units of sodium chloride
(the term "formula unit" is used instead of "molecule" when we refer
to ionic compounds). We can scale these quantities by any number we
choose; for convenience we'll do a scale-up by 6.022 x
1023. And, since 6.022 x 1023 is the number of
particles in one mole, we can convert these unwieldy numbers to
moles.

C. Relating Moles to Mass
To continue with the sodium chloride example: what
would be the mass of 2 moles of NaCl? If we wanted to make 2 moles of
NaCl, what masses of Na and Cl2 would be required?
Recall that the atomic weights (masses) of individual atoms
are expressed in terms of their masses relative to an atom of
carbon-12 having a mass of exactly 12 amu. Let's determine the mass
of a mole of sodium-23 atoms.
One sodium-23 atom has a mass 1.9158 times the mass of a carbon-12
atom (as determined by mass spectroscopy). Thus, one mole of
sodium-23 atoms will have a mass 1.9158 times the mass of a
mole of carbon-12 atoms, or
12.000 g x 1.9158 = 22.990 g/mol Na-23 atoms
Similarly, one beryllium-9 atom has a mass only 0.75102 times as
much as the mass of a carbon-12 atom. Thus one mole of beryllium-9
atoms will have a mass 0.7510 times as much as the mass of a mole of
carbon-12 atoms, or
12.000 g x 0.75102 = 9.0122 g/mol Be-9 atoms
The mass in grams of 6.022 x 1023 atoms of an element
is called the molar mass of that element. For elements, the
molar mass is an amount in grams that is numerically equal to the
atomic weight of the element.
D. Formula Weights and Molar Masses of Molecules and Ionic
Compounds
- Cl2 is a molecule that is made up of two chlorine
atoms. Chlorine has an atomic weight of 35.45. A mole of
Cl2 molecules would contain two moles of chlorine
atoms, each mole weighing 35.45 g. Thus the mass needed to obtain
Avogadro's number of Cl2 molecules is equal to 70.90 g.
The molar mass of Cl2 is 70.90 g/mol.
- NaCl is an ionic compound. Sodium has an atomic weight of
22.990, and chlorine an atomic weight of 35.45. A mole of NaCl
contains a mole of sodium ions and a mole of chloride ions. Since
a mole of sodium has a mass of 22.990 g and a mole of chlorine has
a mass of 35.45 g, the molar mass of NaCl is 58.44
g/mol.
- P4S3 is a molecular compound. Phosphorus
has an atomic weight of 30.97, and sulfur has an atomic weight of
32.07. A mole of P4S3 contains four moles of
phosphorus atoms and three moles of sulfur atoms. Thus its
molar mass is
4 mol P (30.97 g P/mol P) + 3 mol S (32.07 g S/mol S) = 220.09
g P4S3 /mol P4S3
E. Calculations Involving the Molar Mass
- How many moles of P4S3 are there in
0.125 g of P4S3?
(0.125 g P4S3) x (1 mol
P4S3 /220.09 g
P4S3)
= 5.68 x 10-4 mol P4S3
- What mass of P is needed to prepare 2.00 moles of
P4S3?
(2.00 mol P4S3) x (4 mol P/ mol
P4S3) x (30.97 g P /mol P)
= 247.8 g P needed
F. Calculating Percent Composition From the Molar Mass
Let's calculate the percentage of P in
P4S3. We know from Section D that there are 4
moles of P in one mole of P4S3. Now one mole of
P has a mass of 30.97 g, and one mole of P4S3
has a mass of 220.09 g. So the % P in P4S3
is:
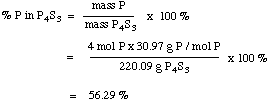
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_8.html
Last modified October 3, 2000 by M. F. Richardson
© Brock University, 2000