Lecture 9
Percent Composition and Empirical Formulas
Readings: Sections 3.7-3.8 in Kotz and Treichel 4th ed.
A. Expressing the Constant Composition of a Compound.
Recall Proust's Law of Constant Composition: the ratio of masses of
the elements pure samples of a given compound does not vary. We can
express this in three ways:
- By atom (or mole) ratios of the elements, e.g.
P4S3 has an atom ratio of 4 atoms of
phosphorus to 3 atoms of sulfur in each molecule, or 4 moles of
phosphorus to 3 moles of sulfur in each mole of
P4S3 molecules.
- By mass ratios, e.g. 56.29 g of P react with 43.71 g of S to
form 100.00 g of P4S3.
Thus the mass ratio of P:S for P4S3 is
56.29 g P to 43.71 g S, or more simply,
1.288 g P to 1.000 g S.
- By percent composition of the compound. Again, taking
P4S3 as the example:
If 56.29 g of P react with 43.71 g of S to form 100.00 g of
P4S3, then the percentages of P and S are:
% P in P4S3 is 56.29% P
% S in P4S3 is 43.71% S
B. Deducing the Chemical Formula from % Composition
We have learned to calculate the molar mass of a
compound according to this schematic process:
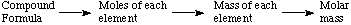
We also know how to calculate the % of a given element in the
compound when the formula is known:
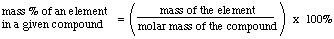
However, chemists often make new compounds and need to determine
their chemical formulas from experimental data. Now surely if we can
get % composition from the chemical formula, we should be able to
reverse the process and get the formula from the composition. In
other words, we will go from % composition to the formula by
following this schematic process:
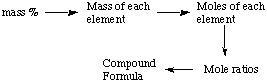
Example. Suppose you have a compound of phosphorus and
oxygen. You analyze it and find that it contains 43.64 % P and 56.36
% O. What is its empirical formula? (The empirical formula is the
formula that has the lowest whole-number ratio of the atoms.)
Solution. Step 1. Mass % to mass of each element. Let's
assume that we have 100 g of the compound. Then we know from the
percentages that 100 g of the compound will contain 43.64 g P and
56.36 g O.
Step 2. Mass to moles of each element.
(43.64 g P) x (1 mol P/30.97 g P) = 1.409 mol P
(56.36 g O) x (1 mol O/16.00 g O) = 3.523 mol O
Step 3. Moles to mole ratio of the elements. Now we
determine the mole ratio: divide the moles of oxygen by the moles of
phosphorus.
3.523 mol O/1.409 mol P = 2.500 mol O/mol P
That is, for every mole of phosphorus, we have 2.500 moles of
oxygen.
Step 4. Mole ratio to empirical formula of the compound.
The empirical formula must contain whole numbers of atoms. The ratio
2.5:1 is the same as the ratio 5:2. Thus, the empirical
formula of our unknown compound is
P2O5.
C. Empirical Formula vs. Molecular Formula
In Part B above, we determined that the simplest
formula for our compound is P2O5. Is this the
actual formula of an individual molecule of
P2O5? One way to find out is to experimentally
determine the molar mass of our compound by techniques that will be
discussed later in the course.
Suppose that we conduct an experiment and learn that the molar
mass of our compound is 283.9 g/mol. We calculate the molar mass of
P2O5:
molar mass P2O5 = (2 mol P) x (30.97 g
P/mol) + (5 mol O) x (16.00 g O/mol)
= 141.94 g/mol if the formula is
P2O5
However, the experimental molar mass is 283.9 g/mol, exactly twice
the calculated molar mass:
283.9 g/mol (exptl.) / 141.94 g/mol (calcd.) = 2.000
This means that we have to have twice as many atoms of P and O to
make a molecule. Thus the molecular formula of our compound is
P4O10.
D. Hydrates
Many compounds are known that can exist as an
anhydrous form (anhydrous means "without water") and as one or
more hydrated forms. One such compound is cobalt(II) chloride.
In this particular case, the colours of the hydrated and anhydrous
forms are dramatically different:
CoCl2 (anhydrous cobalt(II) chloride) is
deep blue
CoCl2.6H2O
(cobalt(II) chloride hexahydrate) is pink
Note the way the formula is written for the hexahydrate.
"CoCl2.6H2O"
means that each mole of CoCl2 combines with 6 moles of
H2O to form one mole of the new compound,
CoCl2.6H2O. (See
p. 99 in Kotz and Treichel, or look on Chapter 3 of your CD-ROM to
see a movie of the color changes.)
The empirical formulas of many hydrates can be determined by
heating the hydrate until all the water is driven off. If the sample
is weighed before and after heating, the weight loss is due to water,
and the sample weight after heating is the weight of the anhydrous
compound. The empirical formula can then be determined:
mass of water lost ---> moles of water
mass of anhydrous compound ---> moles of compound
mole ratio water/compound ---> formula of hydrate
(See Example 3.13, p. 137 in Kotz and Treichel for a worked
example.)
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_9.html
Last modified September 29, 2000 by M. F. Richardson
© Brock University, 2000