

This assignment is most easily worked if you have a clear view of the groups of the periodic table, classified according to the orbitals which are being filled. In the s-block (Groups 1 and 2), the s orbitals are filling; in the p-block, the p-orbitals are filling, and so on. Then elements are easily identified according to their electron configuration:
s-block: electronic configuration has 1 (Group 1) or 2 (Group 2) electrons in an orbital whose principal quantum number is the same as the period number.1. Example. An element has the following electronic configuration: [Kr] 4d10 5s2 5p4.Example: Potassium has an [Ar] 4s1 configuration; it is in Period 4 (electron is in a 4s orbital) and Group 1 (only one electron in 4s)p-block, periods 2 and 3: electronic configuration has (Group number - 10) electrons in the ns and np orbitals. There are two electrons in the ns orbital, and the remaining electrons go into the np orbitals (n is the period number).Example: Phosphorus has a [Ne] 3s23p3 configuration. Thus phosphorus is in Period 3 (electrons are in the 3s and 3p orbitals), and belongs to Group 15 [number of valence electrons = 2 + 3 = 5 = 15 (group number) -10].p-block, periods 4 and higher: electronic configuration has ten electrons in (n-1)d orbitals, two electrons in the ns orbital, and the remaining electrons in np orbitals. (NOTE: at this point, the d-electrons are no longer valence electrons and are not used in bonding.)Example: Selenium has a [Ar] 3d10 4s24p4 configuration. Thus selenium is in Period 4 (electrons are in the 4s and 4p orbitals), and belongs to Group 16 (number of electrons in the configuration beyond the preceding rare gas = 10 + 2 + 4 = 16).d-block: electronic configuration has zero, one, or two (usually two) electrons in an ns orbital, where n is the period number. The remaining electrons are in the (n-1)d orbitals.Example: Technetium has a [Kr] 4d5 5s2 configuration. Thus it is in period 5 (electrons are in the 5s orbitals), and belongs to Group 7 (number of electrons in the configuration beyond the preceding rare gas = 5 + 2 = 7).f-block: the f-block is not considered in first-year chemistry, but the (n-2)f orbitals are being filled as these series of 14 elements is completed.
(a) What period does it belong to? Answer: 5 (outer s and p electrons have principal quantum number n = 5)2. Hints.(b) What is its group number? Answer: 16 (10 + 2 + 4 electrons = 16 electrons beyond the preceding noble gas, Kr)
(c) What kind of element is it? Answer: metalloid (Once you know the period and the group number, you can find the element: it lies at the intersection of the period and group numbers. Look at the periodic table on the inside front cover of Kotz and Treichel for color-coded element classifications)
(d) How many unpaired electrons are there in an atom of this element? Answer: 2. (Fill in the three p-orbitals with 4 p -electrons according to Hund's Rule).
(a) If you are given the complete electronic configuration, you can either count all the electrons to find the atomic number and thus the element, or you can look at the outer electrons and place the element as described in the section on the periodic table at the beginning of these hints.3. - 6. Hints and Examples. These questions all deal with diamagnetism and paramagnetism, and the specific number of unpaired electrons in an element or ion. You will have to write the electronic configuration for each element or ion, and use Hund's rule as you fill in the electrons in a partly-filled subshell.(b) In part (b), the electrons following the preceding noble gas are shown. Get the period from the principal quantum number of the outermost (s and/or p) electrons, and the group number from the total number of electrons beyond the preceding rare gas. Find the intersection of the group number and the period to get the element.
Hund's rule: the most stable arrangement of electrons in a subshell is the one that gives the maximum number of unpaired spins.
Diamagnetic substances are not attracted to a magnet; all their electrons are paired.
Paramagnetic substances are attracted to a magnet; they have at least one unpaired electron per atom.
Example: Give the number of unpaired electrons in each of the following ions. State whether each is paramagnetic or diamagnetic. Mo has an "anomalous" configuration, 4d5 5s1, and so does Rh ( 4d8 5s1).7. - 8. Hints. If the only thing you care about is the trends (and not the reasons for the trends), then just locate the elements in the periodic table and remember the following:Tc2+, Tc5+, Mo3+, Rh3+, I-
Solution: There are three steps to this question. (1) Start with the electronic configurations of each of the atoms in a zero oxidation state. (2) Add or remove electrons as necessary to obtain the charge. Remember that (ns) electrons are removed before (n-1) d electrons. (3) Place the electrons correctly in the orbitals.
Steps (1) and (2)
Tc: [Kr] 4d5 5s2. Therefore Tc2+ has [Kr] 4d5 and Tc5+ has [Kr] 4d2
Mo: [Kr] 4d5 5s1 . Therefore Mo3+ has [Kr] 4d3
Rh: [Kr] 4d8 5s1 . Therefore Rh3+ has [Kr] 4d6
I: [Kr] 4d10 5s2 5p5 . Therefore I- has [Kr] 4d10 5s2 5p6
Step (3): configurations of atoms and of ions are shown
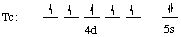
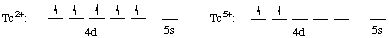


NOTE CAREFULLY:
Electrons are first removed from the ns orbital (5s), then from the (n-1)d orbital (4d).
When electrons are taken from the d orbitals, electrons are first removed from orbitals in which there are two electrons (application of Hund's rule).
Answers:
Tc2+ has 5 unpaired electrons and is paramagneticTc5+ has 2 unpaired electrons and is paramagnetic
Mo3+ has 3 unpaired electrons and is paramagnetic
Rh3+ has 4 unpaired electrons and is paramagnetic
I- has no unpaired electrons and is diamagnetic
The trend in ion sizes down a group is that ions of the same charge are smaller at the top of the group and larger at the bottom (Li+ is smaller than Na+)
The key concept in this question is to be sure you have a series of isoelectronic ions. If you don't, you can still reason your way to a correct answer.
Example: Which of the following ions has the smallest radius? Which the largest?(a) K+ (b) Rb+ (c) Br- (d) Na+The first thing to do is look at the numbers of electrons and the electronic configurations:K+ has 18 electrons, Rb+ has 36 electrons, Br- has 36 electrons, Na+ has 10 electrons. So we aren't dealing with isoelectronic species here! But look at the positions of these elements in the periodic table.
We know that Rb+ is larger than K+, and that K+ is larger than Na+. (This is the observed trend; in quantum mechanical terms, we say that the principal quantum number of the outermost electrons is greater for rubidium than for potassium than for sodium, and the principal quantum number determines the size of the orbital.)
Now, Br- has only 35 protons in its nucleus but Rb+ has 37, so the bromide ion is larger than the rubidium ion since there are fewer protons to attract the electrons.
Answer: Thus Na+ is the smallest ion and Br- is the largest.
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM_1P80_Assign_10.html
Last revised: August 20 2000 by M. F. Richardson
© Brock University, 1999