The ore contains 15.35% CaMoO4; therefore 15.35 g CaMoO4 in 100 g ore:So now that we have defined the information we've been given, we can use dimensional analysis to solve the problem:
CaMoO4 has 1 mole of Mo in each mole of CaMoO4, or 95.94 g Mo in 200.01 g of CaMoO4.
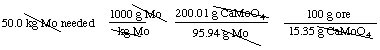
= 6.79 x 105 g or 679 kg of ore is needed to yield 50.0 kg
of molybdenum metal