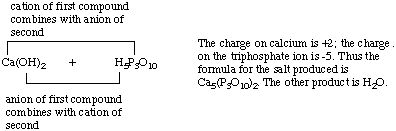Hints for Assignment 5
1. Hints. You will be able to understand chemistry more easily
if you can recognize compound types without having to look them up. So
here are some hints:
Acid: the simplest (Arrhenius) definition of an acid
is that it is a compound containing hydrogen, which dissolves with water
to produce H+ ions. Formulas of most acids have hydrogen as
the first element, e.g. sulfuric acid, H2SO4.
Base: a compound that dissolves in water to produce OH-
ions. Metal hydroxides are bases. So are ammonia and organic amines, which,
although they do not have OH- in their formulas, react with
water to produce hydroxide. For example:
NH3 + H2O
==> NH4+
+
OH-
Soluble bases include hydroxides of the Group 1A cations, along
with ammonia and many organic amines. Sr(OH)2 and Ba(OH)2
are slightly soluble. Other metal hydroxides are generally insoluble.
Strong and weak acids and bases: strong acids
and bases ionize completely in water; weak acids and bases ionize only
a small amount. See Table 5.2, Kotz and Treichel, p. 193.
Acid oxide: compound of oxygen with a nonmetal; reacts with water
to form an acid
Basic oxide: compound of oxygen with a metal; reacts with water
to form a base (metal hydroxide). Soluble oxides include oxides
of the Group 1A cations, along with SrO and BaO which are slightly soluble.
Other metal oxides are generally insoluble.
Soluble and insoluble salts: A salt is a compound consisting
of cations combined with anions other than oxide or hydroxide.
Soluble salts: almost all salts of sodium, potassium,
and ammonium ions; all acetates, chlorates, perchlorates, and nitrates;
most chlorides and sulfates
Insoluble salts: carbonates, phosphates, and sulfides are insoluble
except for salts containing ammonium or Group 1A cations.
2. Hints. See above hints; also Table 5.4, p. 184 in Kotz and Treichel
4th edition.
3. Hints. Note that when an Arrhenius acid reacts with an Arrhenius
base, a salt and water are produced. The reaction type is called metathesis
or double displacement.
Example. Complete and balance the following equation
for the reaction between calcium hydroxide and triphosphoric acid:
Ca(OH)2 + H5P3O10
==>
Solution. First predict the products of reaction. Determine the
charges on the cation and anion so that you can combine them to produce
the correct formulas for the products.
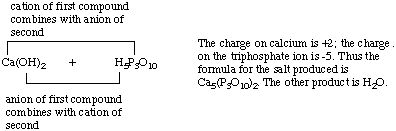
Now write the unbalanced equation:
Ca(OH)2 + H5P3O10
==> Ca
5(P3O10)2
+ H2O
The right hand side has 5 calcium ions and 2 triphosphate ions, so 5 calcium
hydroxides and 2 triphosphoric acids are required on the left. The 10 hydroxide
ions from calcium hydroxide and 10 hydrogen ions from triphosphoric acid
combine to form 10 water molecules.
5 Ca(OH)2 + 2 H5P3O10
==> Ca
5(P3O10)2
+ 10 H2O
4. Hint. The reactions in this question are metathesis reactions.
See Question 3 above and use the solubility rules to determine the soluble
and insoluble products.
5. Guidelines for balancing two types of redox equations There
are no general rules that always work for any kind of equation, but the
following guidelines may be helpful.
1. Combination reactions. A combination reaction is
said to occur when two elements combine to make a compound. These can often
be balanced "by inspection."
Example 1: Na + P4 ==>
Na3P
Here, phosphorus occurs as P4 molecules so balance the P's first
(4 Na3P will be formed). Now the 4 Na3P on the right
require 12 Na on the left to balance.
Balanced: 12 Na + P4
==> 4 Na3P
Example 2. Y + O2 ==>
Y2O3
In this reaction of yttrium with oxygen, the oxygen occurs as diatomic
molecules but the product has 3 atoms of oxygen in each formula unit. We
could balance with fractional numbers (2 Y + 1.5 O2 ==>
Y2O3), but whole numbers are preferred. Thus we double
all the coefficients and the balanced equation is: 4 Y + 3 O2
==> 2 Y2O3
2. Combustion reactions. Many compounds of hydrogen with nonmetals
such as C, B, and Si, burn in air to produce the nonmetal oxide (CO2,
B2O3, SiO2, etc.) and water. The rules
for balancing reactions such as these are given on pp. 152-3 in Kotz and
Treichel, and examples are shown for combustion of hydrocarbons.
6. Hints and examples
1. Reaction of a basic oxide with water.Example. Tl2O
is a basic oxide with Tl in the +1 oxidation state. Thus the formula for
the base is TlOH.
2. Reaction of an acidic oxide with water. These are easiest
if you determine the oxidation number of the nonmetal in the oxide and
connect it to the corresponding acid. Example. P4O6
contains P(III); the two acids of phosphorus are phosphorous acid and phosphoric
acid; phosphorous acid contains P(III) and has the formula H3PO3;
thus the formula for reaction of P4O6 with water
is H3PO3.
7. Example with hints. Give the oxidation number of chromium in
each of the following compounds or ions:
(a) Cr2O72-
(b) KCr(SO4)2 (c) Cr(NO3)3
(d) Cr2(CH3CO2)4
(e) Cr2O5
Answers: (a) +6; (b) +3; (c) +3; (d) +2; (e) +5
Keys to solution:
-
Recognize the formulas and charges for ions other than chromium. Oxide,
sulfate, nitrate, and acetate are represented above.
-
Remember that the oxidation number of chromium refers to each atom of chromium.
Example.
In Cr2O5 each oxygen has an oxidation number of -2,
so each Cr must have an oxidation number of +5.
-
See Example 5.7 on p. 206 and the Table at the top of p. 207, Kotz and
Treichel, for additional examples and guidelines.
8. Hints. See Tables 5.4 and 5.5 in Kotz and Treichel, pp. 209-210,
and Example 5.8, p. 211.
Note: oxidizing agents are chemical species or compounds,
not an individual atom in the species or compound. For example, in the
reaction I2 + 2 S2O32-
==> 2 I- + S4O62-:
I2 (not I) is reduced; it is the oxidizing agent
S2O32- (not S) is oxidized; it is the
reducing agent
Note that the oxidation number of sulfur in S4O62-is
not an integer; it has the fractional value of 2.5!
Back
to Chem 1P80 Home Page
Back
to Assignment Schedule
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM_1P80_Assign_5.html
Last revised: August 20 2000 by M. F. Richardson
© Brock University, 1999