Hints for Assignment 7
1. Hints. (a) Total Calories = Calories from fat + Calories from
protein + Calories from carbohydrate
= mass of fat (9 cal / g) + mass of protein (4 cal / g) + mass
carbohydrate (4 cal/g)
(b) 1 cal = 4.184 J; Therefore 1000 cal = 1 kcal = 1 Cal = 4184 J =
4.184 kJ
(c) % energy from fat = 100 x Calories from fat / total Calories
2. Hints. See worked example on p. 248, Kotz and Treichel 4th editon.
Conversion factors are shown above for part (b).
3. Hints. See Example 6.3 in Kotz and Treichel 4th edition
4. Worked example. When 1.000 kg water at a temperature of 23.1
C is mixed with 2.500 kg water at an unknown temperature, the final temperature
of the resulting mixture is 29.7 C. What was the initial temperature of
the second sample of water?
Solution. heat gained by 1.000 kg water = - heat lost
by 2.500 kg water
Let T2 be the initial temperature for the 2.500
kg water
heat gained by 1.000 kg water = (1000 g) (4.184 J / g-K) (29.7 - 23.1)
K = 2.76 x 104 J (only 2 s.f. are justified - why?)
heat lost by 2.500 kg water = (2500 g) (4.184 J / g-K) (29.7 - T2
) K
Therefore 2.76 x 104 J = - 10460 (29.7 - T2)
- 2.64 = 29.7 - T2
T2 = 32.3 C
Note that the temperature change is Tfinal- Tinitial
for both samples. The final temperatures are the same, the initial
temperatures are different. Also remember that the temperature difference
in K is the same as the temperature difference in degrees C.
5. Worked example. A piece of rhodium weighing 187 g was heated
to 99.7 C and dropped into 142 g of water at a temperature of 16.1 C. What
will be the final temperature of the system once the metal and water have
come to thermal equilibrium? The specific heat of rhodium is 0.243 J/g-K.
The solution to this problem is set up the same way as above:
heat lost by the rhodium = - heat gained by the water.
If we let T be the final temperature, and remember that the heat capacity
of water is 4.184 J/g-K, then
(187 g Rh) (T - 99.7) (0.243 J/g-K) = - (142 g water) (T - 16.1) (4.184
J/g-K)
Multiply through, cancel units, solve for T:
45.44 T - 4530 = -594.1 T + 9565
T = 22.0 C
6. Hints. Note that the chemical substance (element or compound)
is a solid its melting point, and that it is heated beyond its boiling
point. Calculate the following heats separately and add them to get the
final answer:
-
heat needed to melt the solid
-
heat needed to raise the temperature of the liquid from the melting point
to the boiling point
-
heat needed to vaporize the liquid
-
heat needed to raise the temperature of the vapor (gas) from the boiling
point to the final temperature
Remember you don't have a mole of the element or compound and
watch
your units! See Example 6.4 in Kotz and Treichel, 4th edition, for
a worked example. (Note: in Example 6.4, heat has to be added to raise
the temperature of the solid up to the melting point. This term does not
appear in Question 6 because the initial temperature of the solid is the
temperature at the melting point).
7. Hints. Calculate the heat lost by the metal as it cools from
its starting point to 0 C. Then remember it takes 333 J to melt each gram
of ice. Calculate the mass of ice melted.
8. Worked example. The enthalpy change for the formation of solid
sodium bromate from the elements is -334.1 kJ.
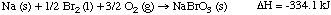
(a) Is this reaction endothermic or exothermic? Answer: exothermic
(negative sign on delta H means that energy is released)
(b) What is the enthalpy change for the following reaction?
4 Na (s) + 2 Br2 (l) + 6 O2 (g) --->
4 NaBrO3 (s)
Answer: We now form four moles of NaBrO3 so four times
as much energy is released, or -1336.4 kJ
(c) What is the enthalpy change for the following reaction?
2 NaBrO3 (s) ---> 2Na (s) + Br2 (l) +
3 O2 (g)
Answer: Now we are decomposing 2 moles of sodium bromate back to
the elements. Formation of sodium bromate was exothermic; decomposition
is therefore endothermic. thus the enthalpy change is 2 x (334.1 kJ) =
668.2 kJ.
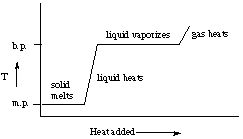
9. Hint. See the figure on p. 253 of Kotz and Treichel, 4th edition.
Make a rough plot of how the temperature of your compound changes as heat
is added to it. Remember:
-
while the solid is melting, the temperature stays constant at the melting
point
-
once the solid is melted, additional heat raises the temperature of the
liquid
-
when the liquid begins to boil, the temperature stays constant at the boiling
point
-
when all the liquid has vaporized, additional heat raises the temperature
of the vapor
Back
to Chem 1P80 Home Page
Back
to Assignment Schedule
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM_1P80_Assign_7.html
Last revised: August 20 2000 by M. F. Richardson
© Brock University, 1999
