Hints for Assignment 9
1. Hints. See middle of p. 301 in Kotz and Treichel for an example
of converting wavelengths to frequencies and to energies.
2. Example. Hydrogen atoms absorb energy so that electrons can
be excited to the n=3 energy level. Electrons then undergo these transitions:
(a) n = 3 ---> n = 2 (b) n = 3 ---> n = 1 (c) n = 2 ---> n
=1
(i) Which transition produces a photon with the least energy?
(ii) Which transition produces a photon with the highest frequency?
(iii) Which transition produces a photon with the shortest wavelength?
Solution. This question is most easily solved by using
a graphical representation of the energy levels in hydrogen. Sketch the
energy levels with decreasing spacing between them to represent the dependence
of energy on - 1/n2. Then draw arrows to represent the transitions.
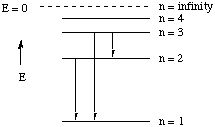
(i) From the diagram it is easily seen that the transition involving
the least energy is the one with the shortest arrow (n = 3 ---> n = 2).
(ii) Energy and frequency are directly related. Therefore the highest-energy
transition is also the one with the highest frequency (n = 3 ---> n = 1).
(iii) Freqency and energy are inversely related to wavelength (see question
1). Therefore high frequencies and high energies correspond to short wavelengths;
low frequencies and low energies to long wavelengths. So the transition
with the shortest wavelength is also the one with the highest energy (n
= 3 ---> n = 1).
3. Example. An atom of some element X has 3 electronic transitions,
A, B, and C. Suppose that it has been found that the wavelength for transition
A is 4.724 x 10-9 m and the wavelength for transition B is 1.525
x 10-8 m. What is the wavelength for transition C?
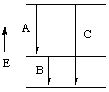
Solution. The key to this question is to recognize that
wavelengths are NOT additive but energies ARE additive. Thus
Energy of transition C = Energy of transition A + Energy of
transition B
So it is necessary to get the energies of transitions A and B given the
wavelengths, just as was done in question 1.
Energy of transition C = 4.2053 x 10-17 J + 1.3024
x 10-17 J
= 5.508 x 10-17 J
Thus the wavelength is 3.607 x 10-9 m, or 3.607 nm
NOTE: The wavelength for transition C is shorter than the wavelength
of transitions A and B, as expected since the wavelength of the transition
is inversely related to the energy.
4. Hint. The quantum number n gives you the shell (1, 2, 3, 4, etc.)
and the quantum number l gives you the shape of the orbital (s,
p, d, f, etc.) See the table at the bottom of page 316 in Kotz and Treichel
and the section entitled "Useful Information from Quantum Numbers" on p.
317.
5. Hint. Review the possible values of quantum numbers. A valid
set of quantum numbers cannot have an impossible value for one of the quantum
numbers. Thus:
n = 0, l = 0, and ml = 0 is an invalid set
because n cannot be zero;
n = 2, l = 2, ml = 0 is an nvalid set because
l
must be a positive integer less than n;
n = 2, l = 1, ml = - 2 is an invalid set because the
absolute value of ml must be less than l .
6. Hints. This is a "summary question" on the chapter, similar to
Exercise 7.7 and to questions 78 and 79 at the end of Chapter 7, pp. 329-330
in Kotz and Treichel. Much useful information is contained in Table 7.1
on p. 318.
Back
to Chem 1P80 Home Page
Back
to Assignment Schedule
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM_1P80_Assign_9.html
Last revised: August 20 2000 by M. F. Richardson
© Brock University, 1999

