1. (18 marks)
(a) (3 marks) Write the formula of the compound formed between:
lithium and oxygen Li2O
magnesium and nitrogen Mg3N2
aluminum and iodine AlI3
(b) (3 marks) Round the following calculations to the proper number of significant figures:
9.2837+ 198.7 + 4.39 = 212.3737 212.4
(437.2 x 11.3579) / 907.62 = 5.471093497 5.471
(166.449 - 146.23) / 123.789 = 0.1633344 0.1633 (166.449 - 146.23 = 20.22; 4 s.f.)
(c) (3 marks) Write formulas for the following compounds:
cobalt(II) nitrate Co(NO3)2
potassium sulfate K2SO4
ammonium monohydrogen phosphate (NH4)2HPO4
(d) (3 marks) Name the following compounds:
NH3 ammonia
N2O5 dinitrogen pentaoxide
FeCl2 iron(II) chloride
(e) (6 marks) State Dalton's Law of Multiple Proportions. Show how it applies to the compounds ClO2 (52.56% Cl) and Cl2O (81.59% Cl).
When 2 elements form two different compounds, the mass ratio of the elements in one compound is a small whole-number ratio of the mass ratio of the elements in the other compound.
ClO2: 52.56 g Cl / 47.44 g O = 1.108
Cl2O: 81.59 g Cl / 18.41 g O = 4.432Cl:O mass ratio in Cl2O / Cl:O mass ratio in ClO2
= 4.432 / 1.108 = 4.000Thus Cl2O has 4 times as much Cl for a given mass of O as ClO2.
(Note: The 4:1 ratio of the Cl:O mass ratios in these two compounds is what led Dalton to the idea that one of these compounds contained four times as many atoms of Cl per atom of O as the other compound.)
2. (10 marks) Fill in the blanks in the following questions:
(a) Consider the ion ![]() The
number of protons in this ion is 29. The number of neutrons is
34. The number of electrons is 26. The atomic number is
29. The mass number is 63. The element is Cu
(copper).
The
number of protons in this ion is 29. The number of neutrons is
34. The number of electrons is 26. The atomic number is
29. The mass number is 63. The element is Cu
(copper).
(b) A mole of oxygen atoms contains 6.022 x 1023 atoms. A mole of O3 molecules contains 6.022 x 1023 molecules. A mole of O3 molecules contains 18.066 x 1023 atoms. A mole of O3 molecules has a mass of 47.998 grams.
3. (10 marks) (a) A compound contains 63.11 % C, 12.36 % H, and 24.53 % N. What is its empirical formula?
Get moles of each element, then determine the mole ratio. Assume 100 g of the compound, so that you have 63.11 g C, 12.36 g H, 24.53 g N. 1 extra digit shown in calculations below.
63.11 g C x (1 mol C / 12.011 g C) = 5.2543 mol C
12.36 g H x (1 mol H / 1.0079 g) = 12.263 mol H
24.53 g N x (1 mol N / 14.0067 g) = 1.7513 mol N
Moles of N is the smallest number; get other ratios relative to N:
5.2543 mol C / 1.7513 mol N = 3.000 mol C / mol N
12.263 mol H / 1.7513 mol N = 7.002 mol H / mol N
Therefore the empirical formula is C3H7N.
(b) If the experimental molar mass is 114.2 g/mol, what is the molecular formula?
C3H7N has a formula weight of 3*12.011 + 7*1.0079 + 14.0067 = 57.10 g/mol. This is exactly half of the experimental molar mass (114.2 / 57.10 = 2.00), so the molecular formula is C6H14N2.
4. (10 marks) A sample was analyzed for the percentage AlCl3. The sample was dissolved in water, excess AgNO3 was added to precipitate AgCl, and the AgCl was filtered and weighed. If 0.3500 g of the sample yielded 0.6358 g of AgCl, what is the % AlCl3 in the sample? The balanced equation is:
Molar masses: AlCl3 133.3 g/mol; AgCl 143.3 g/mol
First determine how much AlCl3 could be produced from 0.6358 g of AgCl:
= 0.19714 g AlCl3 (1 extra digit shown; all extra digits carried in calculator)
% Al = 0.19714 g AlCl3 / 0.3500 g x 100 = 56.33 %
5. (12 marks) Phosphorus reacts with chlorine to produce phosphorus pentachloride according to the following equation:
(a) How many moles of chlorine are required to react with 36.0 grams of phosphorus?
= 2.91 mol Cl2
(b) What mass of phosphorus pentachloride can be produced?
= 242 g PCl5
(c) If 221 g of PCl5 are actually obtained, what is the percent yield of PCl5?
(221 g obtained / 242 g theoretically possible ) x 100
= 91.3 %
6. (18 marks)
(a) (10 marks) Balance the following equations, using the lowest possible whole-number coefficients:
2 CO + O2 ---> 2 CO2
2 Fe + 6 HCl ---> 2 FeCl3 + 3 H2
2 I2 + 5 O2 ---> 2 I2O5
Al2(SO4)3 + 6 NaOH ---> 2 Al(OH)3 + 3 Na2SO4
2 C2H2 + 5 O2 ---> 4 CO2 + 2 H2O
(Note: coefficients of "1" are not normally written. If there is no coefficient, it is understood to be "1").
(b) (3 marks) Metallic nickel has a density of 555 lb/ft3. What is its density in g/cm3? (Conversions: 1 lb = 454 g; 1 ft = 30.48 cm)
(555 lb/ft3) x (454 g/lb) x (1 ft / 30.48 cm )3
= 8.90 g / cm3
NOTE: (1 ft / 30.48 cm)3 = (1 ft / 30.48 cm) x (1 ft / 30.48 cm) x (1 ft / 30.48 cm)
(c) (5 marks) The mass of 0.4700 mol of a compound with the formula MCl4 is 158.3 g.
(i) What is the molar mass of MCl4?
158.3 g / 0.4700 mol = 336.8 g/mol
(ii) What is the atomic weight of M?
336.81 g/mol MCl4 - 4 mol Cl x (35.45 g Cl /mol)
= 195.0 g/mol
(iii) What is M? (give the symbol).
The element with atomic weight closest to 195.0 g/mol is Pt (platinum)
7. (10 marks) Answer the following questions using the figures shown here.
NOTE: Each question may have more than one answer; each figure may be used more than once. Write "NONE" if no drawing fits the description.
SCORING: Score = 2 x (# right - # wrong/4)
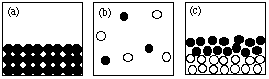
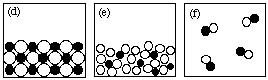
(i) Which drawing(s) represent submicroscopic particles in a sample of an element? a
(ii) Which drawing(s) represent submicroscopic particles in a gas? b, f
(iii) Which drawing(s) represent submicroscopic particles in a sample of a liquid compound? none
(iv) Which drawing(s) represent submicroscopic particles in a sample of a molecular solid? a
8. (12 marks) You are a demonstrator in the CHEM 1P80 class and you need to prepare 4.000 L of a 15.00% (by mass) solution of sodium chloride. Use the graph to help you answer the following questions:
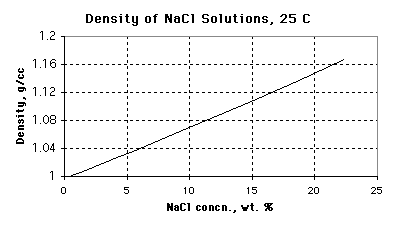
(a) What is the density of a solution that is 15.00 wt. % NaCl?
1.107 +/- 0.003 g / cm3
(Full units are g solution / cm3 solution)
(b) What mass (in grams) of sodium chloride would you take to make up the solution?
mass of solution = (4.00 L solution) x (1000 mL/L) x (1.107 g solution /mL solution) = 4428 g solution
4428 g solution x (15.00 g NaCl /100 g solution)
= 664.2 g NaCl
(c) What mass (in grams) of water would you take to make up the solution?
mass water = mass solution - mass NaCl
= 4428 g solution - 664.8 g NaCl
= 3764 g water
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Midterm_1A_2000.html
Created October 17, 2000 by M. F. Richardson
© Brock University, 2000