Midterm 1B: Questions and Answers
1. (18 marks)
(a) (3 marks) Write the formula of the compound formed between:
sodium and sulfur Na2S
aluminum and fluorine AlF3
calcium and phosphorus Ca3P2
(b) (3 marks) Round the following calculations to the proper
number of significant figures:
9527.3 + 575.4847 + 2.356 = 10105.1407 10105.1
(1.535 x 36.0045) / 165.72 = 0.33349570 0.3335
(54.785 - 49.186) / 186.2073 = 0.03055197 0.03055 (54.785 -
49.186 = 5.599; 4 s.f.)
(c) (3 marks) Write formulas for the following compounds:
cobalt(II) carbonate CoCO3
aluminum nitrate Al(NO3)3
ammonium sulfate (NH4)2SO4
(d) (3 marks) Name the following compounds:
CH4 methane
P4O10 tetraphosphorus pentaoxide
FeCl3 iron(III) chloride
(e) (6 marks) State Dalton's Law of Multiple Proportions. Show how
it applies to the compounds IF (86.98% I) and IF5 (57.19%
I).
When 2 elements form two different compounds, the
mass ratio of the elements in one compound is a small whole-number
ratio of the mass ratio of the elements in the other compound.
IF: 86.98 g I / 13.02 g F = 6.680
IF5: 57.19 g I / 42.81 g F = 1.336
IF mass ratio / IF5 mass ratio = 6.680 / 1.336 =
5.001 = 5.000 within experimental error (3rd decimal place).
Thus IF has 5 times as much I for a given mass of F as
IF5.
(Note: The 5:1 ratio of the I:F mass ratios in these two compounds
is what led Dalton to the idea that one of these compounds contained
five times as many atoms of F per atom of I as the other
compound.)
2. (10 marks) Fill in the blanks in the following questions:
(a) Consider the ion 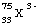 The
number of protons in this ion is 33. The number of neutrons is
42. The number of electrons is 36 . The atomic number
is 33. The mass number is 75. The element is As
(arsenic).
The
number of protons in this ion is 33. The number of neutrons is
42. The number of electrons is 36 . The atomic number
is 33. The mass number is 75. The element is As
(arsenic).
(b) A mole of boron atoms contains 6.022 x 1023
atoms. A mole of B12 molecules contains 6.022 x
1023 molecules. A mole of B12 molecules
contains 72.264 x 1023 atoms. A mole of
B12 molecules has a mass of 129.732 grams.
3. (10 marks) A compound contains 68.55 % C, 8.63 % H, and 22.83 %
O. What is its empirical formula?
Get moles of each element, then determine the mole
ratio. Assume 100 g of the compound, so that you have 68.55 g C, 8.63
g H, 22.83 g O. 1 extra digit shown in calculations below.
68.55 g C x (1 mol C / 12.011 g C) = 5.7073 mol
C
8.63 g H x (1 mol H / 1.0079 g) = 8.562 mol H
22.83 g O x (1 mol O / 15.999 g O) = 1.4270 mol O
Moles of N is the smallest number; get other ratios relative to
O:
5.7073 mol C / 1.4270 mol O = 4.000 mol C / mol
O
8.562 mol H / 1.4270 mol O = 6.000 mol H / mol O
Therefore the empirical formula is
C4H6O.
(b) If the experimental molar mass is 140.2 g/mol, what is the
molecular formula?
C4H6O has a formula weight of
4*12.011 + 6*1.0079 + 15.999 = 70.09 g/mol. This is exactly half of
the experimental molar mass (140.2 / 70.09 = 2.000), so the molecular
formula is C8H12O2.
4. (10 marks) A sample was analyzed for alum,
KAl(SO4)2. The sample was dissolved in water,
excess BaCl2 was added to precipitate BaSO4,
and the BaSO4 was filtered and weighed. If 0.4720 g of the
sample yielded 0.6416 g of BaSO4, what is the %
KAl(SO4)2 in the sample? The balanced equation
is
2 BaCl2 (aq) + KAl(SO4)2
---> KCl (aq) + AlCl3 (aq) + 2 BaSO4 (s)
Molar masses: KAl(SO4)2 258.2 g/mol
BaSO4 233.4 g/mol
First determine the mass of
KAl(SO4)2 that can be produced from 0.6416 g
BaSO4:
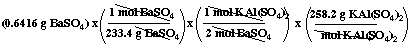
= 0.35489 g KAl(SO4)2 (1 extra
digit shown; all digits carried in calculator)
% Al = 0.35489 g KAl(SO4)2 /
0.4720 g x 100 = 75.19%
5. (12 marks) Arsenic reacts with oxygen to produce diarsenic
trioxide according to the following equation:
As4 + 3 O2 ---> 2
As2O3
Molar masses: As4 299.7 g/mol; O2
32.00 g/mol; As2O3 197.9 g/mol
(a) How many moles of oxygen are required to react with 125 grams
of arsenic?
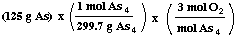
= 1.25 mol O2
(b) What mass of diarsenic trioxide can be produced?
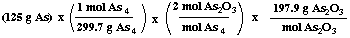
= 165 g
(c) If 158 g of As2O3 are actually obtained,
what is the percent yield of As2O3 ?
158 g obtained/ 165 g possible x 100
= 95.8%
6. (18 marks)
(a) (10 marks) Balance the following equations, using the lowest
possible whole-number coefficients:
2 SO2 + O2 ---> 2
SO3
2 Ag + 2 HNO3 ---> 2
AgNO3 + H2
I2 + 7 F2 ---> 2 IF7
2 AuCl3 + 3 Na2S --->
Au2S3 + 6 NaCl
2 C3H6 + 9 O2
---> 6 CO2 + 6 H2O
(Note: coefficients of "1" are not normally written. If there is
no coefficient, it is understood to be "1").
(b) (3 marks) Gold metal has a density of 19.3 g/cm3.
What is its density in lb/ft3? (Conversions: 1 lb = 454 g;
1 ft = 30.48 cm)
19.3 g / cm3 x (1 lb / 454 g) x (30.48
cm / ft)3 = 1.20 x 103 lb/ft3
NOTE: (30.48 cm / ft)3 = (30.48 cm / ft) x (30.48 cm /
ft) x (30.48 cm / ft)
(c) (5 marks) The mass of 0.2750 mol of a compound with the
formula MO3 is 63.76 g.
(i) What is the molar mass of MO3?
63.76 g / 0.2750 mol = 231.9 g/mol
(ii) What is the atomic weight of M?
231.9 g/mol MO3 - 3 mol O x (15.9994 g O
/mol O) = 183.9 g/mol M
(iii) What is M? (give the symbol).
The element with atomic weight closest to 183.9 is
W (tungsten)
7. (10 marks) Answer the following questions using the figures
shown here.
NOTE: Each question may have more than one answer; each figure may
be used more than once. Write "NONE" if no drawing fits the
description.
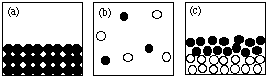
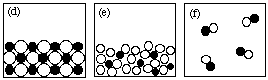
(i) Which drawing(s) represent submicroscopic
particles in a sample of a heterogeneous mixture? (c)
(ii) Which drawing(s) represent submicroscopic particles in a
sample of an ionic solid? (d)
(iii) Which drawing(s) represent submicroscopic particles in a
sample of a compound? (d), (f)
(iv) Which drawing(s) represent submicroscopic particles in a
gaseous mixture? (b)
8. (12 marks) You are a demonstrator in the CHEM 1P80 class and
you need to prepare 7.000 L of a 20.00% (by mass) solution of sodium
chloride. Use the graph below to help you answer the following
questions:
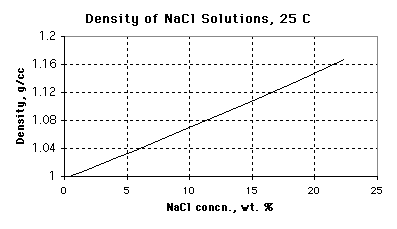
(a) What is the density of a solution that is 20.00
wt. % NaCl?
1.147 +/- 0.003 g / cm3
(Full units are g solution / cm3
solution)
(b) What mass (in grams) of sodium chloride would you take to make
up the solution?
mass solution = (7.00 L solution ) x (1000 mL/L) x
1.145 g solution /mL solution
= 8015 g solution
8015 g solution x (20.00 g NaCl /100 g solution)
= 1603 g NaCl
(c) What mass (in grams) of water would you take to make up the
solution?
mass water = mass solution - mass NaCl
= 8015 g solution - 1603 g NaCl
= 6412 g water
Back
to Midterm Exams
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Midterm_1B_2000.html
Created October 17, 2000 by M. F. Richardson
© Brock University, 2000
![]() The
number of protons in this ion is 33. The number of neutrons is
42. The number of electrons is 36 . The atomic number
is 33. The mass number is 75. The element is As
(arsenic).
The
number of protons in this ion is 33. The number of neutrons is
42. The number of electrons is 36 . The atomic number
is 33. The mass number is 75. The element is As
(arsenic).

