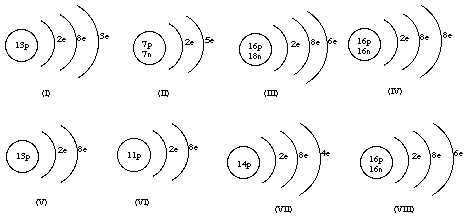
1. Match the atom diagrams with the symbols.

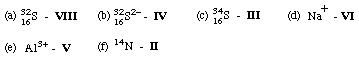
2. Draw atom diagrams for the first 17 elements in the periodic table.
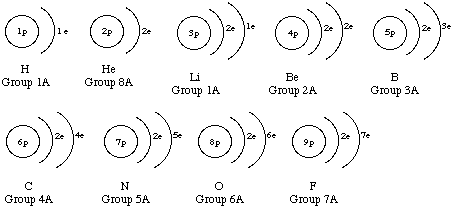
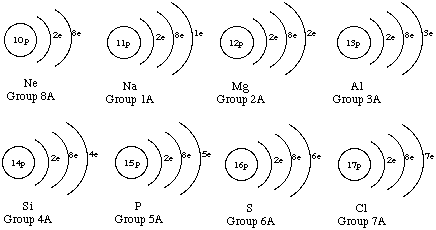
3.Look at your diagrams in question 2 and look at a periodic table. What relationship is there between the group number in the periodic table and the number of valence electrons in each atom in your diagram?
Valence electrons are those in the outer shell. For elements in the first two groups, the number of valence electrons is the same as the group number. For elements in groups 13-18 (except for He), the number of valence electrons is the group number minus 10.
4.(a) How many valence electrons does each of these elements have? Br, K, As, Pb, Ba, Mn
Br has 7, K has 1, As has 5, Pb has 4, Ba has 2, Mn has 7.
(b) What is the atomic number of each of these elements? Br, K, As, Pb, Ba, Mn
Br is 35, K is 19, As is 33, Pb is 82, Ba is 56, Mn is 25
(c) What are the names of the elements corresponding to these atomic numbers? 15, 20, 26, 38, 53?
15 is phosphorus, 20 is calcium, 26 is iron, 38 is strontium, 53 is iodine.
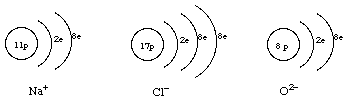
5.Draw diagrams to show how these ions are formed by adding or removing electrons from the atoms: Na+, Cl-, O2-, P3+, P5+, P3-

This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Study_Guide_Ans_Pt1.html
Created October 1, 2000 by M. F. Richardson
© Brock University, 2000