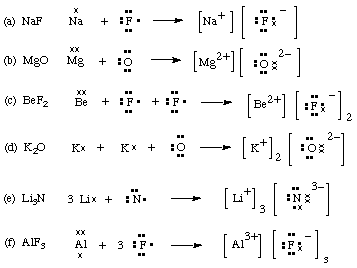
1. Which of these compounds are ionic? Which are covalent?
|
(a) KF ionic |
(i) Li3P ionic |
|
(b) NF3 cov. |
(j) N2O3 cov. |
|
(c) MgCl2 ionic |
(k) K2S ionic |
|
(d) HCl cov. |
(l) BH3 cov. |
|
(e) CO2 cov. |
(m) CH4 cov. |
|
(f) CaH2 ionic |
(n) PCl3 cov. |
|
(g) P4O10 cov. |
(o) SF2 cov. |
|
(h) NH3 cov. |
(p) FeO ionic |
2. Draw diagrams to show how the following ionic compounds are formed from the atoms of corresponding elements.
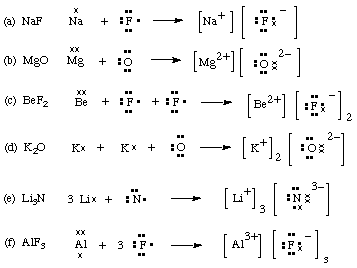
3. Four of the following Lewis structures are wrong (they have the wrong number of electrons, do not obey the octet rule and/or do not obey the rule described for elements 1-4). Which are they? Why is each wrong?


H3O has 9 electrons about O; N2 has two too many electrons; FS has 7 electrons around S; Be still has one electron in its outer shell.
4. Draw diagrams to show how the following covalent compounds are formed from the atoms of corresponding elements.
5. Why do you think it is unlikely that a compound such as NaF2 would be discovered? Do you think that H2F could be formed? [Can you draw acceptable Lewis structures for these?]
Lewis structures that obey the usual rules cannot be drawn for these molecules. Once Na+F&endash; has formed and each element is "satisfied," there is no room for another F that needs an electron. Similarly, in covalent HF, each element has achieved its desired electronic configuration and there is no room for another H.
6. Write formulas for the compounds which can be formed between the given ions:
|
(a) Na+ and Br- NaBr |
(g) Cr3+ and SO42- Cr2(SO4)3 |
|
(b) Ca2+ and Cl- CaCl2 |
(h) H+ and SO42- H2SO4 |
|
(c) NH4+ and SO42- (NH4)2SO4 |
(i) Li+ and N3- Li3N |
|
(d) Be2+ and NO3- Be(NO3)2 |
(j) Ca2+ and N3- Ca3N2 |
|
(e) Fe3+ and ClO3- Fe(ClO3)3 |
(k) A13+ and O2- Al2O3 |
|
(f) Fe2+ and PO43- Fe3(PO4)2 |
(l) Mg2+ and OH- Mg(OH)2 |
7. (a) Why do you think that the sodium ion is Na+ rather than, say, Na- or Na2+? What about Ca2+? A13+? O2-? F-? N3-? S2-? Li+? K+? Cl-?
Na+ has lost its valence electron and its second shell now has 8 electrons. Removing another electron to make Na2+ requires removing an electron from the core shells; this can't happen. Adding an electron to neutral Na to make Na- gives 2 electrons in the outer shell instead of the 8 demanded by the octet rule.
Ca2+, Al3+, Li+, and K+ form when the atoms lose all their valence electrons. O2-, F&endash;, N3-, S2-, and Cl- are formed when enough electrons are added to the valence level to give an octet (one extra electron for F and Cl, two for O and S, three for N).
(b) Can you generalize your answer to question 7(a) so that you can use the periodic table to help you remember what some common ions are (and their charges)?
Many common cations are formed when metals lose all of their valence electrons. If an atom with n valence electrons loses them, it will have a charge of +n. Many monoatomic anions are formed when nonmetals gain enough electrons to complete an octet. If a nonmetal has n valence electrons, it will need to gain (8-n) to give a full octet.
8. H2, F2, Cl2, O2, and N2 are diatomic molecules. He, Ne, Ar, Kr, and Xe are monoatomic. Draw electron dot structures to show why hydrogen, fluorine, chlorine, oxygen, and nitrogen are diatomic but helium, neon, argon, and xenon are monoatomic.
Atoms of H, F, Cl, O, and N have incomplete octets and share their electrons with another atom to achieve octets. Two oxygen atoms, each with 6 valence electrons, share two electrons with each other and thus form a double bond. A nitrogen atom has to share three of its electrons with another nitrogen, so a triple bond is formed.
He, Ne, Ar, Kr, and Xe already have complete octets and do not need to share electrons with another atom, hence they remain monoatomic.
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Study_Guide_Ans_Pt2.html
Created October 1, 2000 by M. F. Richardson
© Brock University, 2000