
1. A golf ball weighs 45.41 g, and a gross (144) of styrofoam balls weighs 224.6 g.
(a) How much does a dozen golf balls weigh? 544.9 g (0.5449 kg)
(b) If you have a box of golf balls weighing 22.30 kg, how many golf balls are in the box? 491
(c) How much does one styrofoam ball weigh? 1.560 g
(d) How much heavier is a golf ball than a styrofoam ball? 29.11 times as heavy
2. An atom of 198 Pt weighs 16.497 times as much as an atom of 12C.
What is the mass (in amu) of one atom of 198Pt? 197.96 amu
What is the mass (in g) of one atom of 198Pt? 3.287 x 10-22 g
What is the mass (in g) of 1.000 x 1020 atoms of 198Pt? 3.287 x 10-2 g
3. For each of the following elements, calculate: (a) the molar mass; (b) the number of moles of molecules in 10.00 g, (c) the moles of atoms in 10.00 g; (d) the number of molecules in 10.00 g.
|
Element |
Molar mass, g/mol |
Moles of molecules in 10.00 g |
Moles of atoms in 10.00 g |
No. of molecules in 10.00 g |
|
H2 |
2.016 |
4.960 |
9.920 |
2.987 x 1024 |
|
Xe |
131.29 |
7.617 x 10-2 |
7.617 x 10-2 |
4.587 x 1022 |
|
P4 |
123.895 |
8.071 x 10-2 |
3.229 x 10-1 |
4.861 x 1022 |
|
S8 |
256.48 |
3.899 x 10-2 |
3.119 x 10-1 |
2.348 x 1022 |
Note the distinction between moles of molecules, number of molecules, moles of atoms, and number of atoms.
4. How many moles of iron does 0.185 mole of each of the following compounds contain?
|
FeCl2 |
0.185 mol Fe |
|
FeCl3 |
0.185 mol Fe |
|
Fe2(SO4)3 |
0.370 mol Fe |
|
Fe3O4 |
0.555 mol Fe |
|
Fe(NH4)2(SO4)2.6H2O |
0.185 mol Fe |
5. Calculate the formula weights and molar masses for each of the following iron compounds: (atomic weights used: Fe = 55.847; Cl = 35.453; S = 32.06; O = 15.999; H = 1.008; N = 14.007)
|
FeCl2 |
126.753 amu (molar mass = 126.753 g/mol) |
|
FeCl3 |
162.206 amu (molar mass = 162.206 g/mol) |
|
Fe2(SO4)3 |
399.87 amu (molar mass = 399.87 g/mol) |
|
Fe3O4 |
231.539 amu (molar mass = 231.539 g/mol) |
|
Fe(NH4)2(SO4)2.6H2O |
392.13 amu (molar mass = 392.13 g/mol) |
6. How many moles of iron are contained in 2.000 g of each of the following compounds?
|
FeCl2 |
0.01578 mol Fe |
|
FeCl3 |
0.01233 mol Fe |
|
Fe2(SO4)3 |
0.01000 mol Fe (1 mol of compound ---> 2 mol Fe) |
|
Fe3O4 |
0.02591 mol Fe (1 mol of compound ---> 3 mol Fe) |
|
Fe(NH4)2(SO4)2.6H2O |
5.100 x 10-3 mol Fe |
7. What mass of each of the following iron compounds must be taken in order to obtain 0.300 moles of iron?
|
Fe(NH4)2(SO4)2.6H2O |
118 g |
|
FeCl2 |
38.0 g |
|
FeCl3 |
48.7 g |
|
Fe2(SO4)3 |
60.0 g (each mol of compound ---> 2 mol Fe) |
|
Fe3O4 |
23.2 g (each mol of compound ---> 3 mol Fe) |
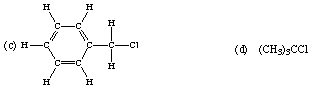
8. How many moles of molecules are contained in 1.000 kg of each of the following organic substances? How many moles of C, H, and Cl atoms are contained in 1.000 kg of each?

(a) Molar mass: C3H7Cl = 78.542 g/mol
1.000 kg = 12.73 moles of molecules
38.20 moles of C atoms
89.12 moles of H atoms
12.73 moles of Cl atoms
(b) Molar mass: C4H6Cl2 = 124.998 g/mol
8.000 moles of molecules
32.00 moles of C atoms
48.00 moles of H atoms
16.00 moles of Cl atoms
(c) Molar mass: C7H7Cl = 126.586 g/mol
1.000 kg = 7.900 moles of molecules
55.30 moles of C atoms
55.30 moles of H atoms
7.900 moles of Cl atoms
(d) Molar mass: C4H9Cl = 92.568 g/mol
10.80 moles of molecules
43.21 moles of C atoms
97.23 moles of H atoms
10.80 moles of Cl atoms
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Study_Guide_Ans_Pt7.html
Created October 1, 2000 by M. F. Richardson
© Brock University, 2000