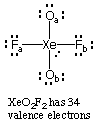
|
 |
Review Chapter 9 for Lewis structures, VSEPR theory, resonance,
bond order, and formal charge.
Example 1. Draw the Lewis structure of XeO2F2. Give the electron pair geometry at each atom, the hybridization of each atom, the formal charge on each atom, and the molecular shape for XeO2F2.
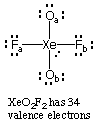
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finally, the molecular shape of XeO2F2 is see-saw
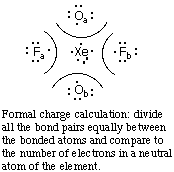
Notes:Example 2. Draw the resonance forms for the cyclic molecule S2N2 (sulfur alternates with nitrogen around the ring).1. The formal charge of Xe is +2 because a neutral Xe atom would have 8 electrons around it, and after dividing the bonding electrons as shown above the Xe atom has only 6 electrons. Similarly, the formal charge of each O is -1 and the formal charge of each F is zero.
2. Get the hybridization from the electron pair geometry. Each electron pair geometry corresponds to a specific hybridization:
Electron pair geometry Hybridization linear sp triangular planar sp2 tetrahedral sp3 trigonal bipyramidal dsp3 or sp3d octahedral d2sp3 or sp3d2
(a) How many sigma bonds are there? How many pi bonds?(b) What is the average S-N bond order in this molecule?
(c) Arrange the following species in order of decreasing S-N bond strength: S2N2, NH2SH, SN+, SN-.
Answers. S2N2 has a total of 22 electrons. There are 4 resonance forms in which all atoms obey the octet rule.
(a) There are 4 sigma bonds and one pi bond in each resonance form. (Whenever there is a multiple bond, one bond is a sigma bond and the other is a pi bond.)
(b) The average S-N bond order is 5/4 = 1.25
(c) Bond strength depends on bond order. Therefore you need the Lewis structures of all four compounds.
SN+ has an S-N bond order of three (triple bond), SN- has an S-N bond order of two, and NH2SH has an S-N bond order of one (single bond).
Thus, the compounds in order of decreasing bond strength are SN+, SN-, S2N2, and NH2SH
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/CHEM_1P81_Assign_1.html
Last revised: 9 January 2001 by M. F. Richardson
© Brock University, 2001