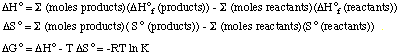1. Predict whether the entropy change for each of the following reactions will have a positive value or a negative value. Calculations are not necessary!
(a) NH4NO3 (s) ---> N2 (g) + 2 H2O (g) + 1/2 O2 (g)Answers:(b) 3/2 N2 (g) + 2 Na (s) ---> 2 NaN3 (s)
(c) N2 (g) + O2 (g) ---> 2 NO (g)
(d) S8 (s) + 8 Cl2 (g) ---> 8 SCl2 (l)
(a) When a solid (low entropy) goes to gases (high entropy), the entropy change is expected to be positive since gases have much higher entropies than solids.(b) Here's the opposite to (a). A gas and a solid combine to produce a solid. The expected entropy change is negative since there are more moles of gaseous reactants than products.
(c) Two moles of gaseous reactants (one mole each of N2 and O2) combine to produce two moles of gaseous products (NO). The entropy change is likely positive since the product contains both nitrogen and oxygen (so there is "mixing" which gives more possibilities for forming products). However, you'd want to confirm this with actual entropy values.
(d) Likely negative because there 8 moles of gaseous Cl2 on the left and only one mole of solid S8, whereas there are 8 moles of liquid SCl2 on the right. This reasoning is based on the more highly disordered nature of the gaseous phase compared either to the liquid or the solid.
2. Hint. See Example 20.1 in your text (p. 921).
3. Example and Hints. (a) Use the data given below and calculate Delta Ho, Delta So, Delta Go, and Kp at 25° C for the reaction:
Cl2 (g) + 3 F2 (g) ---> 2 ClF3 (g)
(b) Calculate Delta G for the reaction at 250 °C.
(c) At what temperature (°C) is Delta G equal to zero? In what temperature range is this reaction product-favored?
Compound Delta Ho formation, kJ/mol So, J/mol.K Cl2 (g) 0 223.1 F2 (g) 0 202.8 ClF3 (g) -163.2 281.6 Hints. Use the following relationships to get the answers to the questions asked.
(a) Answer Watch your units! You should get the following answers for Delta Ho, Delta So, Delta Go, and Kp (if I haven't made a mistake!)
Delta Ho = -326.4 kJ
Delta So = -268.3 J/K
Delta Go = -246.5 kJ at 25 oC
Kp = 1.6 x 1043
(b) Answer Use the values of Delta Ho and Delta So above with the relationship
Delta Go = Delta Ho - T (Delta So),
with T = 250 C (523 K), to get Delta Go = -186.1 kJ.
(c) Answer. Solve
Delta Go = Delta Ho - T (Delta So),
for Delta Go = 0 to get T = 1216.5 K (944 C). Above this temperature the reaction has positive Delta G, so is nonspontaneous.
4. Hints. (But be sure you can explain WHY)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|