(a)
(b)
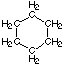
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
|
(a) |

(b) |
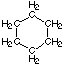
(c) |

(d) |
(e) |
(f) |
|
(g) |
(h) |
(i) |

(j) |
(k) |

(l) |
HINTS.
Questions 1 through 7 make use of the Figure shown above.
1. (a) Give the letters of two compounds that are structural isomers. (g) and (k) OR (k) and (l)
(b) Give the letters of a second pair of compounds that are structural isomers. (c) and (f)(c) Give the letters of two structures that represent the same compound. (g) and (l)
2. Give the letter of a compound that gives a basic reaction to pH paper.
Amines are bases. Therefore the answer is (i)
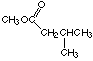
3. Give the names of the acid and alcohol required to produce the ester shown in Figure (g).
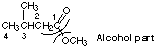
3-methylbutanoic acid and methanol
4. Name all the compounds in the Figure above.
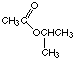
(a) 4-methyl-1-pentyne5. (a) Give the letters of two compounds that produce the same product when they react with hydrogen.(b) The ester is made from 2-propanol and acetic acid. Therefore its name is 2-propyl acetate.
(c) cyclohexane
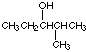
(d) 2-methyl-3-pentanol (not 4-methyl-3-pentanol)
(e) 1,2,4-trichlorobenzene
(f) 4-methyl-2-pentene
(g) methyl 3-methylbutanoate (see the answer to Question 3)
(h) 2,2-dibromo-4-methylbutane
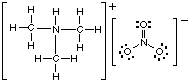
(i) trimethylamine
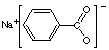
(j) sodium benzoate
(k) hexanoic acid
(l) This is the same compound as (g), just written "backwards" (alcohol part first). It has the same name as (g), methyl 3-methylbutanoate.
There are only two compounds in the Figure that react readily with hydrogen; they are (a) and (f). (a) reacts with two moles of hydrogen to produce 2-methylpentane; (f) reacts with one mole of hydrogen to produce the same product. Thus these must be the correct compounds.(b) Name the reaction product. 2-methylpentane
6. The compound shown in the Figure above reacts with nitric acid to form a salt. Draw the Lewis structure of the salt. Remember to enclose ions in brackets!
7. Draw and name the geometric isomers of compound shown in Figure (f).
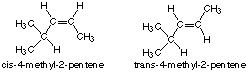
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/CHEM_1P81_Assign_2.html
Last revised: January 18 2001 by M. F. Richardson
© Brock University, 2001