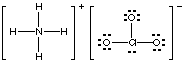 |
 |
|
|
|
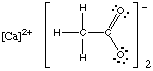
1. Hints. Review Chapter 9, Section 4 on drawing Lewis structures. You will need to remember how to recognize when the compound is ionic and when it is covalent. Examples of Lewis structures for the ionic compounds ammonium chlorate and calcium acetate are shown below.
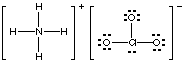 |
 |
|
|
|
2. Hints and an Example.
Review Chapter 13, Section 2 on Intermolecular Forces. The answer to each part of Question 2 will be one of the following:3. Hints. See Question 2 above.ion-ion forcesBefore you can make a decision between dipole-dipole and dispersion forces you will need Lewis structures and shapes. For example consider two compounds with similar formulas, SF4 and XeF4.
ion-dipole forces
dipole-dipole forces
hydrogen bonding forces
induced dipole-induced dipole (dispersion) forces
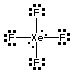
XeF4 has an octahedral electron pair geometry, and a square planar molecular shape.
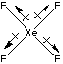
The bond dipoles cancel for XeF4, therefore the molecule is nonpolar and forces between the molecules are of the induced dipole-induced dipole type.
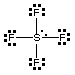
SF4 has a trigonal bipyramidal electron pair geometry, and a see-saw molecular shape.
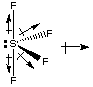
The bond dipoles in the axial positions cancel in SF4, but not the ones in the equatorial positions. Therefore SF4 has a permanent dipole moment and is polar. Attractions between molecules are of the dipole-dipole type.
4. Hints. Review Chapter 13, Section 2 on Intermolecular Forces
(especially Coulomb's Law on p. 586.) See also Example 13.3 on p. 598.
5. Example. Let's estimate the vapor pressure of water at 80o C from the graph on page 603. The key to getting a good estimate lies in accurate measurements with a ruler marked off in millimeters.
The first thing to do is to establish a scale for the graph. Lay your ruler (shown in blue below) with its "zero" exactly on the line for 200 mm Hg. Now you find, if you keep your ruler parallel to the pressure axis (the y-axis), that the line for 400 mm Hg is exactly 13.0 mm distant from the line for 200 mm. That gives us a scale for the drawing, that every 200 mm Hg pressure corresponds to a measured vertical distance of 13.0 mm on the graph, or200 mm Hg pressure/13.0 mm on graph
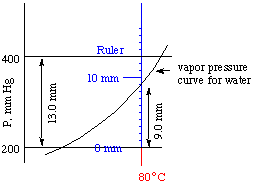
Now lay your ruler along the 80o C vertical line (shown in red), the temperature for which you want the vapor pressure of water. Measure the distance from the 200 mm Hg pressure line to the point where the vapor pressure curve for water crosses the 80o C vertical line. This distance is 9.0 mm.The vapor pressure of water can now be estimated. It is 200 mm Hg plus the additional amount above the 200 mm line:
= 338 mm Hg
This method is much more accurate than an "eyeball" estimate of where the intersection of the vapor pressure curve intercepts the 80o line.6. Hint. See Example 13.5 on p. 604.
7. Hints
8. Hints.
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/CHEM_1P81_Assign_4.html
Last revised: February 1 2001 by M. F. Richardson
© Brock University, 2001