Hints for Assignment 5 CHEM 1P81
Page numbers are to Kotz and Treichel, 4th edition
1. Hints and Example. Use the definitions of molality, weight
percent, and mole fraction:
molality = moles solute / kg solvent
wt. % = mass solute / 100 g solution
(remember that mass of solution = mass of solvent + mass of
solute)
mole fraction of X = moles of X / total moles in the mixture
Example: Find the mole fraction of NaCl and the wt. % of NaCl in
a 1.00 m solution of NaCl in water (fw of NaCl = 58.44 g/mol; of H2O
= 18.02 g/mol).
Answer: The solution contains 1 mole of NaCl in exactly 1000
g solvent. or 58.44 g NaCl in 1000 g water.
Therefore the total mass = 58.44 g NaCl + 1000 g H2O
= 1058.44 g
Wt. % NaCl = 100 * 58.44 g/1058.44 g = 5.52%
Mole fraction = moles NaCl / (total moles, H2O + NaCl)
moles H2O = 1000 g * 1 mol/18.02g = 55.5 moles H2O
mole fraction of NaCl = 1.00 mol NaCl /( 1.00 mol NaCl + 55.5 moles
H2O)
= 0.0177
2. Hints.
(a) Exothermic processes release energy to the surroundings,
endothermic processes take energy from the surroundings. Review Chapter
6: relationship of sign of energy change to endothermic/exothermic reactions,
and page 655.
(b) See page 655, Temperature Effects on Solubility and LeChatelier's
principle
(c) See example on page 651, where the standard enthalpy of formation
of aqueous NaCl is calculated from the standard enthalpy of formation of
solid NaCl and the enthalpy of solution of NaCl.
3. Hints.
This is a Henry's law calculation; Henry's law constants are
found on p. 653, and an example is shown on p. 654.
4. Hints.
Get molality of solution: moles of solute / mass of solvent
in kg. Then use the equation for boiling point elevation, with the appropriate
value of Kb for the solvent (p. 661). See worked example on
p. 662.
5. Hints.
Calculate the total molality of the ions in each case, using
the van't Hoff factor (# of ions in one formula unit of the compound).
Remember that covalent molecules such as CH3OH do not dissociate
into ions, so their van't Hoff factor is 1.
Then recall that the freezing/boiling point is directly proportional
to the total molality of particles present. Solutions with the highest
molality have the greatest boiling point elevations and freezing point
depressions.
6. Hints
1 ppm = 1 g solute in 1,000,000 g solution
= 1 mg solute in 1000 g solution
= 1 mg solute in 1 liter solution if the density of the solution = 1.00
g/mL
So, a solution that is 312 ppm in some solute X contains 312 mg of X
per liter.
7. Hints
Use the equation for osmotic pressure.
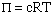
All the variables are known except the concentration c (molarity). Find
c, then use the given volume to determine the number of moles present in
the solution:
molarity x volume = moles
Finally get the molar mass from the moles and the mass of the solute.
8. Hints
1 atm = 760 mm Hg. In terms of water (the tree sap), this corresponds
to
760 mm Hg x 13.1 mm H2O = 9.96 x 103
mm H2O
Now you can determine what the osmotic pressure (in atm) has to be; it's
the height of the tree divided by 1 atm pressure expressed as mm H2O.
9. Hints
Freezing point is determined by molality, vapor pressure by
mole fraction (Raoult's Law). See page 658 for a worked example of Raoult's
law.
10. Hints
How many grams of the salt can be dissolved in the given amount
of water at the lower temperature?
How many grams of the salt can be dissolved in the given amount of water
at the higher temperature?
The difference in these two numbers is how much salt will precipitate
when the solution is cooled.
Back
to Chem 1P81 Home Page
Back
to Assignment Schedule
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/CHEM_1P81_Assign_5.html
Last revised: February 14 2001 by M. F. Richardson
© Brock University, 2001
![]()