1. (6 marks)
(a) When MgCl2, NaCl, CaCl2, and CsCl salts are dissolved in water in equivalent amounts, which one of the four cations will be most strongly hydrated?
(b) What type of intermolecular forces are responsible for the hydration of the cation above? Circle all correct answers.
(c) What type of intermolecular forces must be overcome in converting each of the following from a liquid to a gas?
ion-ion ion-dipole dispersion polar-polar dipole-dipole molecular
O2mercury
CH3I
SF4
2. (4 marks) A unit cell of cuprite, a semiconductor material, is shown below. The oxide ions are in a bcc lattice arrangement. The copper ions shown are completely contained within the unit cell (i.e. they are not shared with an adjoining unit cell).
(a) What is the formula of cuprite?(b) What is the oxidation number of copper?
3. (10 marks)
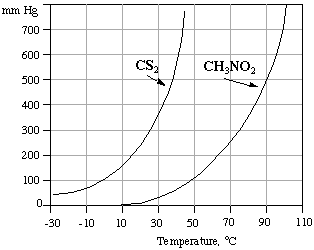
Consider the diagram above and answer the following questions to 2 significant figures.
(a) What are the approximate vapor pressures (in atm) of CS2 and CH3NO2 at 40 oC?
(b) What type of intermolecular forces exist in the liquid phase of each compound?
(c) What is the normal boiling point of CS2? of CH3NO2?
(d) At what temperature does CS2 have a vapor pressure of 350 mm Hg?
(e) At what temperature does CH3NO2 have a vapor pressure of 400 mm Hg?
(f) At 60 oC and 0.658 atm what is the predominaate state
for each compound?
4. (4 marks). An unknown gas composed of homonuclear diatomic
molecules effuses at a rate that is only 0.355 times that of O2
at the same temperature. What is the identity of the unknown gas?
5. (9 marks). Define or explain each of the following:
(a) When considering gases under very high pressures (greater than 1000 atm, for example), what corrections (if any) to the ideal gas law are needed?
(b) Does a cup of water that is slowly evaporating, cool down or warm up? Explain briefly using a particulate view of matter and the distribution of energy curve for individual molecules in this process.
(c) Define miscible and immiscible, citing a specific example of each.
6. (3 marks). Give the relative rates of disappearance of reactants and formation of products for the following reaction:
2 HOF (g) --> 2 HF (g) + O2 (g)
7. (5 marks) A 2.00% solution of H2SO4 in water freezes at -0.796 oC. The normal freezing point of water is 0.000 oC and Kfp is -1.86 oC/m.
(a) Calculate the van't Hoff factor i for the solute above.
(b) From the calculation in part (a), which of the following best represents sulfuric acid in the dilute aqueous solution? Circle your answer.
i) H2SO4 (aq)
ii) HSO4- (aq) and H+ (aq)
iii) SO42- (aq) and 2 H+ (aq)
8. (5 marks) If you add 0.255 g of a solid crystalline compound
that has an empirical formula of C10H8Fe to 11.18
g of benzene, the boiling point rose from 80.10 oC for pure
solvent to 80.26
oC for the solution. Calculate the molar mass
of the compound and determine its molecular formula. Kbp for
benzene = 2.53 oC/m
9. (8 marks) A 250-mL flask containing N2 at 760 mm Hg is connected to a 500-mL flask containing O2 at 380 mm Hg. Both flasks are at 25 oC.
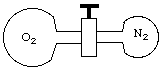
(a) When the valve between the flasks is opened and the gases are allowed to mix, what are the partial pressures of N2 and O2 in the mixture?10. (6 marks) A 1.40 g sample of polyethylene, a common plastic, is dissolved in enough benzene to give exactly 100.0 mL of solution. The measured osmotic pressure of the solution is 1.86 mm Hg at 25 oC. Calculate the molar mass of the polymer.(b) What is the total pressure?
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/Midterm_Exam_1_2000.html
Created November 10, 2000 by M. F. Richardson
© Brock University, 2000