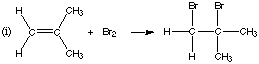
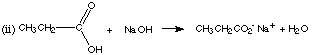
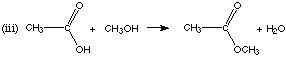
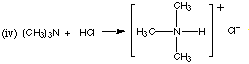
1. (8 marks) Complete the following equations, giving the structures of the organic products. If there is no reaction, state "no reaction."
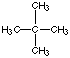
2. (10 marks) (a) (6 marks) Draw three structural isomers of alkanes having the formula C5H12. Name them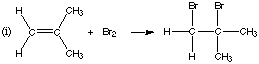
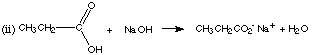
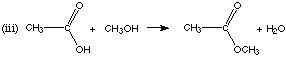
|
|
|
 |
|
|
(isopentane) |
(neopentane) |
(b) (4 marks) Draw structures for two geometric isomers of an alkene having the formula C4H8. Name them.
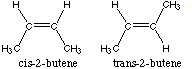
3. (14 marks) (a) (2 marks) Give the ideal values of angles a-d in allyl thiocyanate, whose Lewis structure is below.
(b) (3 marks) In the diagram, write the hybridizations of each atom (other than hydrogens) below the atom.
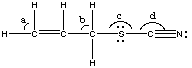 |
Angle a = 120o (C is sp2-hybridized)
Angle b = 109.5o (C is sp3-hybridized) Angle c = 109.5o (S is sp3-hybridized) Angle d = 180o (C is sp-hybridized) |
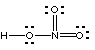
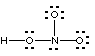
(c) (9 marks). Students have written the Lewis structures shown below for nitric acid, HNO3. At least one of them is correct; the others are wrong. If the Lewis structure is correct, say so. If not, state the reason(s) why it is wrong.
Not correct; 10 electrons around N violates the octet rule |
Not correct; too many electrons in total (26) |
Not correct; wrong connectivity for nitric acid |
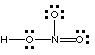
Correct. This is the correct connectivity; octets are achieved about each N and O atom with 24 electrons available for bonding. |
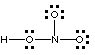
Not correct; 6 electrons around N violates the octet rule |
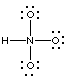
Not correct; too many electrons in total (26). Also wrong connectivity for nitric acid |
4. (11 marks) (a) (3 marks) Briefly define the following terms:
(i) Triple pointThe point at which three phases (gas, liquid, and solid) coexist in equilibrium with each other.
(ii) Critical temperatureThe temperature beyond which it is impossible to liquefy a gas no matter how much pressure is applied. The pressure at this temperature is called the critical pressure.
(iii) Supercritical fluidA substance at a temperature higher than the critical temperature and a pressure higher than the critical pressure. Under these conditions the fluid has the density of a liquid but the very low viscosity associated with a gas.
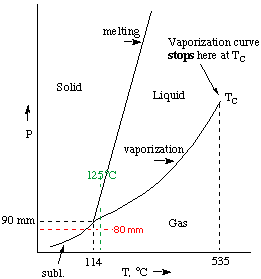
| (b) (6 marks) Use the following information and sketch
the phase diagram for iodine in the box on the right. Label the gas, liquid,
and solid regions, and the vaporization, sublimation, and melting lines.
Iodine has a triple point at 114oC and 90 mm Hg. Its critical temperature is 535o C. The density of the solid is 4.93 g/cm3, while the density of the liquid is 4.00 g/cm3. Diagram isn't to scale. Melting line has a positive slope since the solid is more dense than the liquid (LeChatelier's principle at work here). (c) (2 marks) Use your diagram to fill in the blanks below. Marking scheme: # correct - # wrong. (i) When iodine vapour at 80 mm Hg is cooled, it condenses to a solid (see diagram). (ii) When sufficient pressure is applied to iodine vapour at 125° C, it condenses to a liquid (and ultimately to a solid if pressure continues to increase) . |
 |
5.(12 marks) Below is a plot of boiling point vs. period of the periodic table for the hydrides of Groups 7A (fluorine to iodine) and 6A (oxygen to tellurium) of the periodic table.
(a) (2 marks) On the diagram, sketch where you would expect the boiling points of the corresponding noble gases Ne, Ar, Kr, and Xe, to occur. What kind of intermolecular forces would be important between noble gas atoms?
| Intermolecular forces between noble gas atoms are of the induced dipole-induced dipole type. The atoms are spherical and thus there is no permanent dipole. Polarizability (and thus induced dipole forces) increases with increasing number of electrons in the atom or molecule; thus Ne has the lowest b.p. and Xe the highest. |
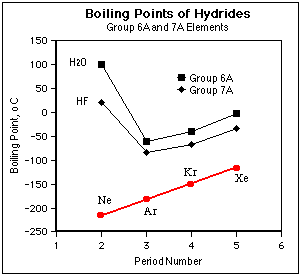 |
(b) (6 marks) Explain, in terms of intermolecular forces, the trend of boiling points from HF to HI.
HF has a very high boiling point compared to the other members of the group because of the great electronegativity difference between H and F. This results in very polar molecules that hydrogen bond to each other more strongly than the HCl, HBr, and HI molecules do.(c) (4 marks) Explain why H2O has a higher boiling point than HF.
Hydrogen bonding is much weaker in the other HX molecules because the electronegativity difference is less, resulting in smaller delta- and delta+ charges on X and H. Hydrogen bonding is weakest for HI because the electronegativity difference is least. However, from HF to HI the number of electrons in the molecules increases, so the polarizability increases. The reason that the boiling points increase from HCl to HI is because the increased dispersion forces (induced dipole-induced dipole forces) more than compensate for the weaker hydrogen bonding.
We saw in the structure of ice that each water molecule forms 4 hydrogen bonds (Donates 2 H's to form H-bonds to two other water molecules, accepts 2 H's on its lone pairs to form two additional H-bonds). However, in HF each HF molecule forms (on average) only 2 H-bonds, one as donor and one as acceptor (see above question). The H-F bond is more polar than the O-H bond because of the greater electronegativity difference, so each individual H-bond is stronger in HF. However, the greater number of H-bonds in water results in a higher boiling point.6. (20 marks) Define or explain:
(a) (3 marks) How are ideal gases different from real gases?
Ideal gas molecules have no volume and do not interact with each other, whereas the molecules of real gases do occupy space and interact by dispersion forces, dipole-dipole interactions, etc. The van der Waals equation for real gases corrects for the finite volume of the gas molecules and for the forces between the molecules.(b) (3 marks) Arrange the following molecules in order of increasing polarity: HF, LiF, CF4, ClF.
LiF is ionic (combination of a metal with a nonmetal) so is the most polar.(c) (2 marks) Give statements of the following laws (words, not equations).CF4 is a tetrahedral (symmetric) covalent molecule so it has no dipole moment and is the least polar.
The electronegativity difference between H and F is greater than the electronegativity difference between Cl and F. Both are linear molecules, so HF is more polar than ClF.
Thus CF4 < ClF < HF <LiF
(i) Avogadro's Law: Equal volumes of gases at the same temperature and pressure contain the same number of molecules(d) (3 marks) Briefly explain the difference between a hydrogen bond and a covalent bond involving hydrogen. Sketch a few molecules of water interacting in the liquid state as part of your explanation.(ii) Boyle's Law: The volume of a gas is inversely related to the pressure exerted by the gas if the temperature and the amount of the gas are kept constant.
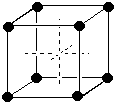
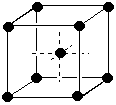
Covalent bonds are formed when two atoms share an electron pair to form a bond within a molecule. It is indicated by a line between two atoms. E.g. H-O-H. If sharing of electrons isn't equal, the bond is polar covalent, but it is still covalent.(e) (3 marks) Use diagrams and briefly explain the difference between simple cubic cells, body-centered cubic cells, and face-centered cubic cells in which there is only a single kind of atom present.Hydrogen bonds are interactions between molecules that involve a partially positively charged hydrogen and a partially negatively charged F, O, N, or Cl.
See Question 5c for the sketch.
 |
 |
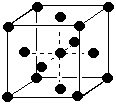 |
| Simple cubic: atoms only at the corners of the cell. Corner atoms shared 8 ways = 1 atom/cell | Body-centered cubic. Atoms at the corners of the cell and one in the middle that belongs entirely to the cell = 2 atoms/cell | Face-centered cubic. Atoms at the corners of the cell and 6 on the centers of the 6 faces that are shared between two cells = 4 atoms/cell |
(f) (2 marks) The figure below represents a cubic unit cell where copper atoms are on the faces and gold atoms are at the corners. What is the formula of this alloy? Show how you arrived at your conclusion.
7. (13 marks) (a) (7 marks) Calculate the density of gaseous sulfur dioxide (SO2) at 55°C and 409 torr.8 Au atoms on the cell corners, each shared 8 ways = 1 Au per cell
6 Cu atoms in the centers of the faces, each face atom shared two ways = 3 Cu atoms per cell.
Answer: Cu3Au.
PV = nRT; density = mass/volume = m/V; moles = n = mass/molar mass = m/M. ThusPV = (m/M)RT
density = m/V = PM/RT
= (409 torr) (1 atm/760 torr) (64.1 g/mol) /[(0.08206 L-atm/mol-K) (328 K)]= 1.28 g/L
(b) (6 marks) If the average speed of an oxygen molecule is 4.28 x 104 cm/s at 25°C, what is the average speed of a sulfur hexafluoride (SF6) molecule at the same temperature?
SF6 has a formula weight of 32.06 + (6)(19.00) = 146.06 g/mol; O2 formula weight is 32.00 g/mol8. (12 marks) (i) Which of the following substances would be expected to have the highest melting point?The kinetic-molecular theory of gases states that all gases at the same temperature have the same average kinetic energy (KE = 1/2 mv2, where m is the mass and v is the velocity).
Thus 1/2 mv2 = 1/2(32.00 g/mol)(4.23 x 104 cm/s)2 for O2
= 1/2 (146.06 g/mol)(v)2 for SF6Solving, we get that the average speed of an SF6 molecule is 1.98 x 104 cm/s.
|
|
|
|
|
|
There is only one nonmolecular compound in the list, and it is the ionic network solid CaO. It has the highest melting point. The forces between the molecules of the other 4 substances (H-bonding, dipole-dipole, dispersion forces) are weak compared to ionic (or covalent) bonds.
(ii) Which one of the following statements is true?
(a) All gas molecules moving with the same velocity have the same kinetic energy.(iii) For a given sample of gas molecules, the average kinetic energy depends only on the value of(b) All gas molecules at the same temperature have the same kinetic energy. (See kinetic molecular theory in your text.)
(c) All gas molecules having the same kinetic energy have the same mass.
(d) As the kinetic energy of a gas molecule is halved, its velocity is also halved.
(e) As the velocity of a gas molecule is doubled, the kinetic energy decreases by a factor of four.
(a) pressure(b) temperature
(c) volume
(d) moles
(e) molar mass
(iv) If 20.0 mL of SO2 (g) and 20.0 mL of Cl2 (g) are allowed to react according to the equation below, what is the total volume of all gases after the reaction if all measurements are done at the same temperature and pressure?
SO2 (g) + 2 Cl2 (g) ---> SOCl2 (g) + Cl2O (g)
(a) 20.0 mL(v) Complete combustion of a 0.20 mol sample of a hydrocarbon, CxHy, gives 0.80 mol of CO2 and 1.0 mol of H2O. What is the molecular formula of the original hydrocarbon?(b) 26.6 mL
(c) 30.0 mL
(d) 40.0 mL
(e) 66.6 mL
This is a limiting reagent problem. Volumes of gases are proportional to moles (PV = nRT). All of the Cl2 will react and there will be 10.0 mL of SO2 remaining, to add to the volumes of SOCl2 and Cl2O.
(a) C3H8(vi) At standard conditions, it was found that 1.12 L of a gas weighed 2.78 g. Its molecular weight is(b) C4H5
(c) C4H8
(d) C4H10
(e) C8H20
There's a 4:1 ratio of CO2 to the hydrocarbon, and a 5:1 ratio of H2O to the hydrocarbon. Thus 4C and 10 H (5 x 2)
(a) 2.78 g/mol(b) 27.8 g/mol
(c) 55.6 g/mol
(d) 111 g/mol
(e) Not enough information is given to solve this problem.
One mole of an ideal gas (and real gases at normal T and P) occupies 22.4 L at STP. 1.12 L is thus 0.0500 moles. 2.78 g/0.0500 moles =- 55.6 g/mol
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/Midterm_Exam_1_2001.html
Created April 5, 2001 by M. F. Richardson
© Brock University, 2001