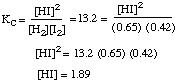
=1.9 to correct s.f.
1. (8 marks) Exactly 100 mL of 0.25 M NaOH was mixed with 160 mL of 0.20 M HCl.
(a) (4 marks) What is the molarity of the NaCl formed?
Answer. This is a limiting reagent problem.
NaOH + HCl ---> NaCl + H2O
moles NaOH = 0.025 moles(b) (4 marks) Calculate the pH and pOH of the solution after mixing.moles HCl = 0.032 moles
therefore NaOH is limiting and 0.025 moles of NaCl can be formed. 0.007 moles of HCl will be in excess.
Total volume is approximately the sum of the volumes of the reactants. Therefore,
molarity of NaCl = 0.025 mol NaCl /(0.100 L + 0.160 L) = 0.096 M NaCl
Answer. Since 0.007 moles of HCl is in excess, the [H+] concentration is0.007 moles /(0.260 L)
= 2.69 x 10-2 M (1 s.f. justified)
pH = - log [H+] = 1.6 (1 s.f.)
pOH = 14 - pH = 12.4 (1 s.f.)
2. (16 marks) Assume that the following reaction is at equilibrium:
2 NOCl (g) --> 2 NO (g) + Cl2 (g) Delta H = +77 kJ
How do the final concentrations compare with those of the original equilibrium, and in which direction does the reaction shift to reach the new equilibrium after the following changes are made?
(a) (4 marks) What happens if you remove some NO? Correct answer shown
in bold below
(i) The NOCl concentration: increases decreases does not change(b) (4 marks) What happens if you remove some NOCl? Circle the correct answer below(ii) The NO concentration: increases decreases does not change
(iii) The Cl2 concentration: increases decreases does not change
(iv) The equilibrium: shifts to the right does not change shifts to the left
(i) The NOCl concentration: increases decreases does not change(ii) The NO concentration: increases decreases does not change
(iii) The Cl2 concentration: increases decreases does not change
(iv) The equilibrium: shifts to the right does not change shifts to the left
(c) (4 marks) What happens if the temperature is raised? Circle
the correct answer below
(i) The NOCl concentration: increases decreases does not change(ii) The NO concentration: increases decreases does not change
(iii) The Cl2 concentration: increases decreases does not change
(iv) The equilibrium: shifts to the right does not change shifts to the left
(d) (4 marks) What happens if you increase the pressure? Circle
the correct answer below
(i) The NOCl concentration: increases decreases does not change3. (14 marks)(ii) The NO concentration: increases decreases does not change
(iii) The Cl2 concentration: increases decreases does not change
(iv) The equilibrium: shifts to the right does not change shifts to the left
(a) (3 marks) Define the terms "Bronsted acid," "Bronsted base," and "conjugate acid-base pair."
Answer A Bronsted acid is a proton donor; a Bronsted base is a proton acceptor. A conjugate acid-base pair is two species that differ by a single proton.In the reaction
H2CO3 is a Bronsted acid becauses it donates a proton to the Bronsted base H2O to form the products. H2O accepts the proton so it is a Bronsted base. Examples of conjugate acid-base pairs are H2CO3/HCO3- and H3O+/H2O.(b) (8 marks) The following reactions and their Ka values allow you to determine the relative acid strengths of four Bronsted acids and the relative base strengths of their conjugate bases.
|
|
|
|
|
|
|
|
|
(i) Based on the Ka values shown, arrange the four Bronsted acids in order of their acid strengths:Answer
|
|
||
H3O+ |
HCO2H |
H2CO3 |
NH4+ |
(ii) Arrange the four Bronsted bases in order of their base strengthsAnswer
|
|
||
NH3 |
HCO3- |
HCO2- |
H2O |
(c) (3 marks) Use the information above and determine whether the following reaction proceeds to the right or not. Explain your reasoning. HCO2- + NH4+ --> HCO2H + NH3
Answer
HCO2- + NH4+ ---> HCO2H + NH3 Base 1 Acid 1 Acid 2 Base 2 weaker base weaker acid stronger acid stronger base From parts (a) and (b): NH3 is a stronger base than HCO2-; HCO2H is a stronger acid than NH4+. Therefore this reaction does not proceed to the right since the direction is stronger acid + stronger base ---> weaker acid + weaker base.
4. (14 marks)
(a) (3 marks) The following reaction has Kc = 13.2 at 700°C.
H2 (g) + I2 (g) --> 2 HI (g)
What is the equilibrium concentration of HI when [H2] = 0.65 M and I2 = 0.42 M?
Answer.(b) (5 marks) What is the H3O+ concentration in a solution that is 0.050 M in hypochlorous acid, HClO2? The Ka for hypochlorous acid is 3.5 x 10-8.
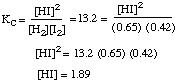
=1.9 to correct s.f.
(c) (6 marks) What is the pH of a solution that is 0.15 M in trimethylamine, (CH3)3N, and 0.20 M in trimethylammonium chloride, (CH3)3NHCl? The Kb for trimethylamine is 7.4 x 10-5.
| 5. (12 marks) At the right is a plot of boiling
point vs. period of the periodic table for the hydrides of Groups VA (nitrogen
to antimony) and VIA (oxygen to tellurium) of the periodic table.
(a) (2 marks) On the diagram, sketch where you would expect the boiling points of the corresponding noble gases Ne, Ar, Kr, and Xe, to occur. What kind of intermolecular forces would be important between noble gas atoms? Answer: Approximate boiling points of rare gases are shown in red. Only intermolecular forces are induced dipole -induced dipole (dispersion forces) |
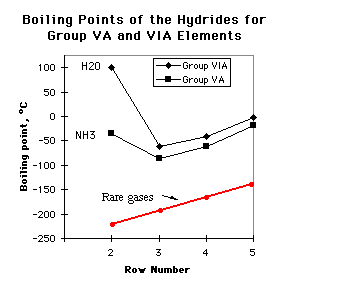 |
(b) (6 marks) Explain, in terms of intermolecular forces, the trend of boiling points from NH3 to SbH3.
Answer Ammonia has the highest boiling point in the series because the N-H covalent bond is quite polar and so the molecules undergo hydrogen-bonding to each other.(c) (4 marks) Explain why H2O has a higher boiling point than NH3.
When ammonia boils, the H-bonds are broken and NH3 molecules go into the gas phase.
PH3, AsH3, and SbH3 boil lower than NH3 because the electronegativities of P, As, and Sb are less than the electronegativity of N and the molecules are less polar than NH3. Thus the dipolar interactions are not as strong as the H-bonds in liquid ammonia.
On going from NH3 to SbH3, the polarizability increases because the number of electrons in the molecules increases. These factors increase the dispersion interactions (induced dipole-induced dipole interactions) so that the boiling point increases from PH3 to SbH3. [Note the b.p. trend for Ne through Xe, where the increase is entirely due to dispersion interactions.]
Answer. There are two reasons. We know from periodic trends that oxygen is more electronegative than nitrogen, so the O-H bond should be more polar than the N-H bond. This will make the H-bonding stronger and more difficult to break in H2O than in NH3.6. (10 marks)The second reason is that we know from the structure of ice that each water molecule is involved in four hydrogen bonds, as shown below. To boil water all four of these must be broken to give H2O molecules. An ammonia molecule is involved in only two water molecules, as shown in part (b).
(a) (4 marks) Which of the following solutes dissolved in 1.0 kg of water would cause the greatest lowering of the freezing point? Explain your answer!
Answer. CH3OH and CH3CO2H are essentially molecular in aqueous solution. NaOH dissociates into two ions (Na+ + OH-), CaCl2 into three ions (Ca2+ + 2 Cl-), and Fe(NO3)3 into 4 ions (Fe3+ + 3 NO3-). The freezing point lowering is proportional to the total number of particles (total molality of particles) in solution. Thus the total molalities are
1.0 mol Fe(NO3)31.5 mol CaCl2
1.5 mol CH3OH
2.5 mol CH3CO2H
1.0 mol NaOH
1.0 m x 4 ions/mol = 4.0 m total particle molality for Fe(NO3)3Thus the freezing point lowering is expected to be greatest for 1.5 m CaCl2 since it has the greatest total molality of particles. (Review the van't Hoff factor if necessary)1.5 m x 3 ions/mol = 4.5 m total particle molality for CaCl2
1.5 m for CH3OH
2.5 m for CH3CO2H
1.0 m x 2 particles per mol = 2.0 m total particle molality for NaOH
(b) (6 marks) When 10.2 g of an unknown compound X is dissolved in exactly 200 g of water, the freezing point was depressed to -2.82 oC. What is the molar mass of X? The freezing point of water is 0 oC and its Kf is -1.86 o kg/mol
Answer. Freezing point depression is related to the molality of the solution,
Thus the molality of the solution = (-2.82 oC / -1.86 oC) = 1.516 mol solute / kg solvent
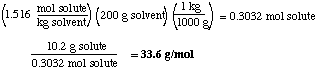
7. (16 marks).
(a) (12 marks) Draw a Lewis structure for each of the following, including non-zero formal charges if any. Give the electron count first (valence shell electrons only). The structures will not be marked if the correct electron count is not given.
(i) CH3CO2H(b) (4 marks) Refer to the Lewis structures in part (a) above and answer the following questions. NOTE: some compounds may not be used at all. If no compound is an appropriate answer to the question, write "None." Marking scheme in each part: Score = # right - # wrong.(ii) Ca(NO3)2
(iii) NH4HSO3
(iv) IF5
(i) Which compound(s), if any, produce 3 ions when dissolved in water? Answer: Ca(NO3)2(ii) Which compound(s), if any, ionize only slightly in water? Answer: CH3CO2H
8. (10 marks)
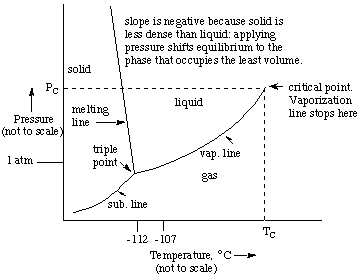
(a) (2 marks) Define the following terms:
(i) Triple point The pressure and temperature at which gas, liquid, and solid phase coexist in equilibrium.(b) Make a rough sketch of a phase diagram for xenon given the following data. The melting point of Xe at 1 atm pressure is -112oC and the normal boiling point is -107oC. The density of solid Xe is 2.7 g/cm3. The density of liquid Xe is 3.52 g/cm3.(ii) Critical temperature The temperature beyond which it is impossible to liquify a gas no matter what pressure is applied. (The pressure at this point is the critical pressure.)
Label the areas in which gas, liquid, and solid will be found. Also label the vaporization line, sublimation line, melting line, critical point, triple point
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem181/Midterm_Exam_2_1998.html
Created March 13, 2001 by M. F. Richardson
© Brock University, 2001