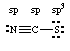
In addition to questions like the ones on the assignments, students can expect to be asked questions of the following types:
define various terms (CFSE, high-spin versus low-spin complexes, magnetic moment, Gouy balance, chelating ligand, fac and mer isomers, ambidentate ligand, octahedral site preference energy, linkage isomers, ionization isomers, Lewis acid, Lewis base, metal complex, and other terms important in this course)The questions below are from past exams in CHEM 2P32. Good student answers (full marks) are shown for some of the questions and are so designated. Otherwise the answers are mine. MFRnomenclature; drawing structures of compounds given their names [e.g. cis-diamminedi-S-thiocyanatoplatinum(II); fac-triamminetrichlorocobalt(III)]; drawing all the isomers possible for a given complex
draw the d-orbitals and label them properly
explain or interpret data
1. Question: Use Lewis structures to explain why the Pt-N-C bond is linear in [Pt(NCS)4]2- but the Pt-S-C bond is bent in [Pt(SCN)4]2-. What are the hybridizations of N and S?
(a) Good student answer, full marks
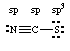
The Lewis structure is shown above with hybridizations. When forming a bond with platinum through the nitrogen, the lone pair of electrons on the nitrogen is oriented 180° from the N-C bond. The Pt forms a coordinate covalent bond with the nitrogen through its lone pair of electrons which gives the Pt-N-C bond a 180o bond angle (linear). The three lone pairs on the sulfur are in a tetrahedral arrangement with the C-S bond. The bond angle between the lone pairs and the C-S bond is therefore about 109.5o. This means that when S is bonding to the platinum through one of its lone pairs, the Pt-S-C bond angle will have to be about 109.5o (bent).(b) Why students lost marks on this question:
(a) Good student answer, full marks.
SO32- has lone pairs so it can bond through either atom. NO3- only has lone pairs of electrons on its oxygen atoms, so can bond only through oxygen. Lewis structures:
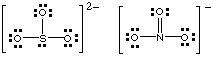
(b) Why students lost marks.
(a) Good student answer with a diagram
| Cu(II) is a d9 ion, and its electronic configuration looks
like this: --->
Since there is space available in an eg orbital, color can result when absorption of radiation necessary to promote an electron from t2g to eg occurs. |
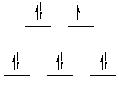 |
| Cu(I) is a d10 ion, and its electron configuration looks like this:
--->
Since there is no space available in eg, t2g electrons cannot be promoted to eg and so no color results.
|
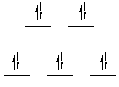 |
(b) Good student answer without a diagram
The Cu(II) cation has the configuration [Ar]3d9. The electron transfers within the split d-orbitals result in changes in energy and the molecule absorbs light in the visible spectrum. Therefore Cu(II) salts are colored. Cu(I) has 10 d-electrons, having the configuration [Ar]3d10. Since the d-orbital is completely filled, no electron transitions occur within the d-orbitals. Since it is electron transitions within the d-orbitals that correspond to the absorption of light in the visible part of the spectrum, an inability for these transitions to occur results in colorless salts.(c) Why students lost marks
Answer.
Compound A is [Co(NH3)5SO4]Br5. Question. Ethylenediamine reacts with K3[Cr(NCS)6] to form two complexes, red compound I and yellow-orange compound II. Each has the empirical formula Cr(en)2(NCS)3, and each gives a deep red solution upon the addition of iron(III). Compound I could be resolved into optical isomers, but compound II could not. Suggest plausible structures for the two compounds and explain your reasoning.Compound B is [Co(NH3)5Br]SO4
The fact that the complexes are neutral means that the NH3 is bonded to cobalt in both compounds; otherwise a solution of ammonia in water would be basic. In any case, ammonia bonds strongly to cobalt(III) and a compound containing both is expected to have all the ammonia molecules coordinated to cobalt.
The white precipitate produced when AgNO3 is added to Compound A is due to the formation of insoluble silver bromide. Thus bromide is ionic. The failure to get a precipitate upon addition of BaCl2 means that the sulfate is coordinated to cobalt.
The white precipitate produced when BaCl2 is added to Compound B is due to the formation of insoluble barium sulfate. Thus sulfate is ionic in Compound B. On the other hand, bromide must be coordinated to cobalt since there is no precipitate when silver nitrate is added.
Answer
6. Question. Draw all the geometric isomers possible for the [Co(NH3)3Cl2(H2O)]+ cation. Circle the ones (if any) that are chiral.
Compound I: cis-[Cr(en)2(NCS)2]SCN Compound II: trans-[Cr(en)2(NCS)2]SCN The red color developed upon addition of iron(III) shows that at least one thiocyanate is uncoordinated. Cr(III) typically has a coordination number of 6. Thus each complex ion is bonded to two ethylenediamine molecules and two two thiocyanates. The two compounds are geometric isomers.
Compound I is the cis-isomer. It lacks a plane of symmetry, and is thus expected to have optical isomers (enantiomers). The second compound has a plane of symmetry so would not have optical isomers.
Answer. There are 3 isomers, 2 mer and one fac. None are chiral.7. Question. Chromium(III) picolinate, Cr(pic)3, is used as a dietary mineral supplement. Draw all the geometrical isomers of Cr(pic)3. Circle the ones (if any) that are chiral. The structure of picolinic acid, Hpic, is shown below.
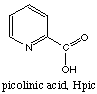
Answer
The picolinate anion is a good chelating agent and forms a five-membered ring with metal ions when it uses its nitrogen and oxygen atoms for coordination. Thus chromium(III) achieves its usual coordination number of 6 with three bidentate ligands. There are two isomers, "mer" and "fac"; both are chiral as there are no planes of symmetry.8. Question. The magnetic moments and colours of a series of cobalt complexes are given below.
(a) Draw appropriate d-orbital splitting diagrams for each.Answers.(b) Calculate the CFSE for the first four. Include the pairing energy.
(c) Explain why complexes of cobalt have so many colours, whereas the complexes of titanium(IV) and zinc(II) are colourless.
Complex Magnetic moment, B.M. Colour [Co(NH3)6]3+ 0.0 yellow [CoF6]3- 4.9 blue [Co(H2O)6]2+ 3.8 pink [CoCl4]2- 3.8 blue [Co(oaph)2] (ligand structure shown below) 1.7 red
The magnetic moment data show that there are no unpaired electrons in the first complex, four in the second, three in the third and fourth, and one in the fifth complex.9. Question. The overall formation constants (B2) for three bis(oxalato) complexes are shown below. Is the stability of the complex related to the CFSE? Explain your answer by showing the CFSE's for each complex (neglect pairing energies). The cobalt complex is high-spin.The first three complexes are octahedral (6 ligands).
[CoCl4]2- and Co(oaph)2 must be four-coordinate since there are not six donor atoms (oaph is a bidentate chelating ligand). They could be either tetrahedral or square. Tetrahedral is most common and would lead to 3 unpaired electrons; one unpaired electron requires square-planar geometry.
Complex d-orbital diagram CFSE [Co(NH3)6]3+ 2.4 DeltaO - 2 P [CoF6]3- 0.4 Deltaoct [Co(H2O)6]2+ 0.8 Deltaoct [CoCl4]2- 0.6 Deltatet [Co(oaph)2] The reason that the complexes of cobalt have so many colors is that Co(II) and Co(III) both have unfilled d-shells. Electronic transitions are possible when electrons in lower-energy orbitals are promoted to vacancies in higher-energy orbitals; energy required for these transitions falls in the visible range.
On the other hand, Zn(II) complexes are colorless because the d-orbitals are completely filled and any transitions to higher energy orbitals correspond to energies outside the visible range; hence no color. Ti(IV) has no d-electrons so there are no transitions possible involving them.
Answer
Complex B2 [Mg(ox)2(H2O)2]2- 2.4 x 104 [Co(ox)2(H2O)2]2- 5.0 x 106 [Ni(ox)2(H2O)2]2- 4.0 x 107
Mg2+ is d0, Co2+ is d7high-spin, and Ni2+ is d8. The corresponding CFSE's are:10. Question. By writing formulas or drawing structures related to the compound of formula VCl2NCS.2en, give examples of the following types of isomers (if they exist for a compound of this composition.
Complex Electronic configuration of metal ion CFSE [Mg(ox)2(H2O)2]2- no d electrons zero [Co(ox)2(H2O)2]2- t2g5eg2 0.8 Deltaoct [Ni(ox)2(H2O)2]2- t2g6eg2 1.2 Deltaoct The stability is related to the CFSE. The higher the CFSE, the greater the stability.
Assume that V is 6-coordinate and that both ethylenediamines are chelated to the vanadium((III) ion.
(a) geometric isomersAnswers(b) optical isomers
(c) linkage isomers
(d) ionization isomers
(a)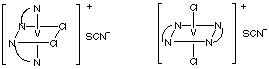
(b)
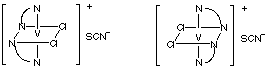
(c)
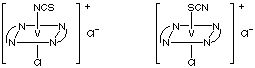
(d) [V(en)2Cl2]NCS and [V(en)2Cl(NCS)]Cl
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/2P32_Typical_Questions_MT1.html
Created January 14, 2001 by M. F. Richardson
© Brock University, 2001