Red = Element commonly found in nature as its oxide
Orange = Element commonly found in nature as its sulfide
Brown = Element commonly found in nature as its soluble halide
Blue = Element commonly found in nature as its carbonate
Green = Element commonly found in nature uncombined
Magenta = Element commonly found in nature as its phosphate
Black. Carbon found as coal, boron as borax, lithium and silicon as silicates*Blank space* in the periodic table: radioactive elements not abundant enough to form minerals.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | La | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi |
Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th U
1. Example. Equilibrium positions have been established for the metathetical (double displacement) reactions shown below. (The reactions do not need to be balanced.) Given that hard acids prefer to combine with hard bases, and soft acids prefer soft bases, determine the order of hardness for the cations Co2+, Fe2+, Ga3+, and Zn2+.
Reaction Equilibrium position FeF2 + CoI2 = FeI2 + CoF2 proceeds to the right CoI2 + GaF3 = CoF2 + GaI3 equilibrium lies on the left ZnI2 + CoF2 = ZnF2 + CoI2 equilibrium lies on the left ZnI2 + FeF2 = ZnF2 + FeI2 equilibrium lies on the left In this question, F- is the hard base and I- the soft base. The reaction given will proceed to the right if the softer metal ion is combined with the hard base and the harder metal ion is combined with the soft base on the left hand side of the equation.
The first reaction shows that Co(II) prefers to combine with the hard base F- and Fe(II) with the soft base I- . Thus Co(II) is harder than Fe(II).
The equilibrium position for the second reaction lies on the left. Thus Ga(III) is harder than Co(II).
The equilibrium position for the third reaction also lies on the left, so Co(II) is harder than Zn(II).
The fourth reaction shows that Fe(II) is harder than Zn(II).
Combining information from the first, second, and fourth reactions we get the order Ga(III) (hardest) > Co(II) > Fe(II) >Zn(II). Data from the third reaction is consistent with this order.
2. Examples. Use the HSAB theory and predict whether each of the following reactions will or will not proceed to the right:
(a) Li2O + HgS = Li2S + HgO3. Examples. Answer the following questions for each ligand whose structure or formula is shown below: (a) What are its donor atoms? Classify each as hard or soft. (b) How many atoms are there in each chelate ring formed?(b) ZnS + Ag2O = ZnO + Ag2S
(c) CdO + PbS = CdS + PbO
(d) Co2S3 + CoO = Co2O3 + CoS
Answers
(a) Hg2+ is much more electronegative than Li+ (2.00 vs. 0.98) and it is larger (116 vs. 90 pm). Also, Hg2+ is usually found as a sulfide in nature whereas Li+ is associated with silicates (O-donors). Thus Hg2+ is expected to be a soft metal ion so the reaction will not proceed to the right.
(b) Ag is more electronegative than Zn (1.93 vs. 1.65), and the silver ion has a lower charge (+1) than the zinc ion (+2). Also the zinc ion is smaller than the silver ion (88 vs 129 pm). These three factors combine to make Ag+ softer than Zn2+. Thus the reaction will proceed to the right.
(c) Pb is more electronegative than Cd (2.33 vs. 1.69) and Pb2+ ion is larger than Cd2+ (133 vs 109 pm). Thus Pb is expected to be softer and the reaction will not proceed to the right.
(d) Co3+ has a higher charge and smaller size than Co2+, so is harder. The reaction will proceed to the right.
(i) Corrin ring as found in vitamin B12, coordinated to cobalt.

Answer. 4 N donors, borderline hard/soft (hard if you have to choose). There are 3 6-membered chelate rings and 1 5-membered chelate ring when this ligand bonds to a metal ion (which sits in the center of the above structure).4. Hints. By now you shouldn't need any hints. You know what the hard and soft donor atoms are, and you know what the hard and soft metal ions are. Choose the ligand with greatest number of soft donor atoms to combine with the soft metal ion, and the ligand with the greatest number of hard donor atoms to combine with the hard metal ion.(ii) Nitrilotriacetate, [N(CH3CO2)3]3-; has been used as a softening agent in detergents.
Answer. Draw the structure. Then you see that there is one N and 3 O donors. O is a hard donor, N is hard or borderline (hard if you have to choose between hard and soft).
There are three 5-membered chelate rings when this ligand bonds to a metal ion.
(iii) Choline, [(CH3)3NCH2CH2OH]+, an important biological compound (especially as acetylcholine).
Answer. Draw the structure. Then you see that there are no lone pairs on nitrogen. This is not a chelating ligand. It can bond only through its oxygen, which is a hard donor.
(iv) [SCH2CH2N=CHCH=NCH2CH2S]2-
Answer. Draw the structure. Nitrogen has lone pairs and the negative charges reside on the sulfurs. This ligand has two soft donor atoms (S) and two hard/borderline donor atoms (N). Three 5-membered chelate rings can form.
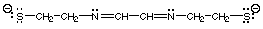
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM_2P32_Assign_10.html
Last revised: April 3 2001 by M. F. Richardson
© Brock University, 2001