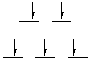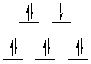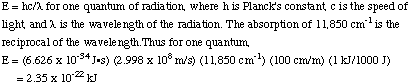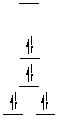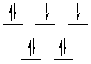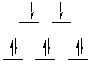CHEM 2P32 Hints for Assignment 4
1. The formulas and magnetic moments of four octahedral complexes are
given below. For each complex, draw the d-orbital splitting diagram and
show the locations of the electrons. Calculate the CFSE for each complex
in terms of the octahedral splitting energy and the pairing energy.
(a) [Fe(H2O)4(OH)2]+,
magnetic moment = 5.92 B.M.
(b) [Cu(NO2)6]4-, magnetic moment =
1.73 B.M.
(c) [Cr(H2O)6]3+, magnetic moment =
3.87 B.M.
(d) [Co(ox)3]3-, magnetic moment = 0 B.M.
2. The octahedral splitting energy for the ion [Ni(en)3]2+
is 11,850 cm-1. Calculate its value in kJ/mol, and determine
the value of the CFSE for [Ni(en)3]2+ in kJ/mol.
Report your answers to 3 significant figures.
3. One of the following two complexes is square planar, the other is
tetrahedral. Which is which? For each, draw the appropriate d-orbital splitting
diagram and show the locations of the electrons.
(a) [Ni(CN)4]2-, magnetic moment = 0
B.M.
(b) [NiBr4]2-, magnetic moment = 2.83 B.M.
4. This question involves a calculation of the octahedral preference energy
for the chloro complexes of Ni2+.
(a) The complex ion [NiCl6]4- has an
octahedral splitting energy of 81 kJ/mol. Calculate its CFSE in kJ/mol.
(b) Assume that the tetrahedral splitting energy is equal to 4/9 of
the octahedral splitting energy, and calculate the CFSE of [NiCl4]2-
in kJ/mol.
(c) What is the octahedral preference energy for Ni2+? (kJ/mol)
5. A researcher recorded the visible spectra of four different complexes
of the same transition metal, [MF6]3-, [M(NH3)6]3+,
[M(H2O)6]3+, and [MCl6]3-.
He labelled the spectra A, B, C, and D, but forgot to write down which
spectrum belonged to which compound.
Look at the spectral data below and tell the poor researcher which spectrum
belongs to which compound.
Spectrum A has a peak at 14190 cm-1 Spectrum B has a peak
at 18200 cm-1
Spectrum C has a peak at 22750 cm-1 Spectrum D has a peak
at 16380 cm-1
Answers
Question 1 Answer
Determine the number of d-electrons in each metal ion. If you
don't have the correct number of d-electrons your answer to this question
will be wrong. Fe(III) has 5 d-electrons, Cu(II) has 9, Cr(III) has 3,
and Co(III) has 6.
Now determine the number of unpaired electrons from the magnetic moment.
Draw the octahedral splitting diagram that shows the correct number of
unpaired electrons. Complex (a) has 5 unpaired electrons, complex (b) has
1, complex (c) has 3, and complex (d) has none.
Recall that each electron in a t2g orbital is stabilized
by 0.4 Deltao, and each electron in an eg orbital is destabilized
by 0.6 Deltao, and calculate the CFSE.
Note that the fourth complex is low-spin (t2g6)
instead of high-spin (t2g4eg2).
Two electrons in the eg orbitals pair with two in the t2g orbitals, thus
leading the correction for the pairing energy in the CFSE.
Question 2 Answer
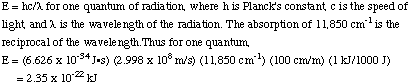
Multiply this number by Avogadro's number to get the octahedral splitting
in kJ/mol.
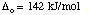
CFSE = 170 kJ/mol
Question 3 Answer
The metal ion in both complex ions is Ni(II), a d8
ion. The crystal field splitting diagrams for tetrahedral and square planar
d8 complexes are shown below. Thus [NiBr4]2-
is the tetrahedral species (magnetic moment corresponds to 2 unpaired electrons),
[Ni(CN)4]2- is square planar (no unpaired electrons).
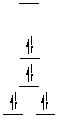
|
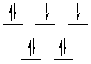
|
|
square planar diagram: magnetic moment is zero
|
tetrahedral diagram; magnetic moment is 2.8 B.M. (2 unpaired electrons)
|
Question 4 Answer
(a), (b) Ni(II) is a d8 metal ion. Octahedral [NiCl6]4-
and tetrahedral [NiCl4]2- have the following splitting
diagrams and crystal field stabilization energies.
Octahedral CFSE = -1.2 x 81 kJ/mol = -97.2 kJ/mol
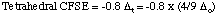
= -0.8 x 4/9 x 81 kJ = -28.8 kJ
(c) Thus, other factors being equal, Ni(II) prefers octahedral coordination
to tetrahedral. The OSPE is the difference between the stabilization energies
in octahedral and tetrahedral coordination,
OSPE = -97.2 - (-28.8) = -68.4 kJ/mol
Question 5 Answer
The peak positions in a set of octahedral complexes of the
same metal ion are expected to be in the same order as the spectrochemical
series as the ligands. The complex whose ligand(s) are highest in the spectrochemical
series will have the highest-energy absorption.
The ligands for the complexes in this question are in the order NH3
> H2O > F > Cl in the spectrochemical series. Thus the spectra
are assigned as follows:
Spectrum A is [MCl6]3-
Spectrum B is [M(H2O)6]3+
Spectrum C is [M(NH3)6]3+
Spectrum D is [MF6]3-
Back
to Chem 2P32 Home Page
Back
to Assignment Schedule
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM_2P32_Assign_4.html
Last revised: January 28 2001 by M. F. Richardson
© Brock University, 2001