Answer. (a). 4: Eu, Eu2+, Eu3+, Eu(OH)3(b) What oxidation states are represented on the diagram?
Answer. There are three oxidation states represented: 0, +2, +3
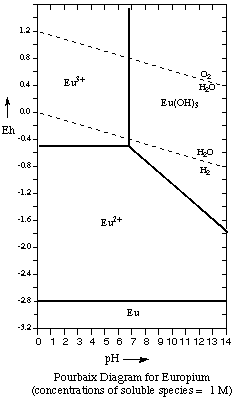
1. The Pourbaix diagram for the element europium is shown below.Redox Reactions and Pourbaix Diagrams
(a) How many chemical species (element, ion, or compound) are shown for this element?2. Use the Pourbaix Diagram and identify which chemical species is stable under the following conditions:Answer. (a). 4: Eu, Eu2+, Eu3+, Eu(OH)3(b) What oxidation states are represented on the diagram?Answer. There are three oxidation states represented: 0, +2, +3
(a) organic-rich waterlogged soils (pH = 4.5, Eh = -0.10 V) Answer: Eu3+3. (a) Which of the species shown in the Pourbaix diagram will react with water at all pH values to generate H2?(b) organic-rich salt water (pH = 9.0, Eh = -0.35 V) Answer: Eu(OH)3
(c) oceans, lakes, streams near the surface of the water (pH = 7.0, Eh = 0.55 V) Answer: Eu3+ / Eu(OH)3
(d) streams and lakes contaminated by acid mine drainage pH = 3.0, Eh = 0.80 V Answer: Eu3+
Answer: Any species whose stability field lies entirely below the H2O/H2 line will react with water to generate hydrogen gas. Thus both Eu metal and Eu2+ willl be oxidized by water and produce H2 gas. Reactions:(b) Which of the species shown will react with water at all pH values to generate O2?2 Eu2+ (aq) + 2 H+(aq)---> 2 Eu3+ (aq) + H2 (g) (acid solution)2 Eu2+ (aq) + 4 OH- (aq) + 2 H2O ---> 2 Eu(OH)3 + H2 (g) (basic solution)
Eu (s) + 2 H+ (aq) ---> Eu2+ (aq) + H2 (g) (acid solution)
Eu (s) + 2 H2O ---> Eu2+ (aq) + H2 (g) + 2 OH- (aq)
Answer: Any species whose stability field lies entirely above the O2/H2O line will react with water to generate oxygen gas. Since no species lies entirely above the O2/H2O line, none will react with water.4. In this question you will obtain 3 pairs of Eh/pH values on the line showing the half reaction Eu3+ + e- ---> Eu2+. Make use of the Nernst equation and the following data: Eo = -0.48 V for Eu3+ + e- ---> Eu2+; Ksp for Eu(OH)3= 2.0 x 10-21.
(a) What is E at pH = 0? Answer: pH 0 corresponds to hydrogen ion activity of 1. Since H+ doesn't appear in the half-reaction, and precipitation of Eu(OH)3 doesn't begin until higher pH values, E = -0.48 V.5. (a) Balance equations for the following half-reactions. For reactions in acidic solution you may use H2O or H+ to balance the equation; for reactions in basic solution you may use H2O or OH- to balance the equation.(b) At what pH does Eu(OH)3 begin to precipitate when the metal ion concentration is 1 M? What is E at this pH?
Answer. The equilibrium constant Ksp refers to the chemical reaction
Eu(OH)3 (s) ---> Eu3+ (aq) + 3 OH- (aq)So when [OH-] = 1.28 x 10-7 M (pH = 7.1), Eu(OH)3 begins to precipitate. The vertical line on the Pourbaix diagram divides the diagram for Eu(III) into two regions: the region below pH 7.1 where Eu3+ is the dominant species, and the region above pH 7.1 where Eu(OH)3 is the dominant species.K = Ksp = 2.1 x 10-21 = [Eu3+][OH-]3
(remember that solids don't appear in equilibrium constant expressions because their concentrations don't change).
When [Eu3+] = 1 M,
2.1 x 10-21 = [1][OH-]3
Thus, at pH 7.1, just as precipitation begins, the Eo value is still -0.48 V
(c) What is the Eu3+ concentration at pH 14? What is E at pH 14?
Answer.
The Eu concentration at pH 14 is found from the Ksp for Eu(OH)3.
Eu(OH)3 (s) ---> Eu3+ (aq) + 3 OH- (aq)Now we have to use the Nernst equation since, as Eu(OH)3 is precipitating, the potential for the reduction of Eu(III) to Eu(II) changes accordingly.K = Ksp = 2.1 x 10-21 = [Eu3+][OH-]3
At pH 14, [OH-] = 1 M so Eu3+ = 2.1 x 10-21 M.
The reaction quotient Q has the same form as the equilibrium constant for the reaction, but refers to the actual (rather than equilibrium) concentrations available. For the reaction Eu3+ + e- ---> Eu2+, Q is [ Eu2+] / [ Eu3+]. Thus,Eu(OH)2 is a soluble compound even at pH 14 so Eu2+ is still 1 M. However, Eu3+ = 2.1 x 10-21 M. Substituting these values into the above equation, we find that E at pH 14 is -1.7 V.
(i) (acidic solution) Rh (s) ---> RhO2 (s)6. Balance the following redox equations by the ion-electron half-reaction method:Answer. The rules for balancing redox half reactions in acid solution are:(ii) (basic solution) Rh (s) ---> RhO2 (s)(i) First balance the number of non-H and non-O atoms in the half reaction given (in black) belowThus for the reaction above,(ii) Next balance the O's by adding water molecules as needed. (in red below)
(iii) Then balance the H's with H+ (in blue below)
(iv) Finally, balance the charge on each side of the equation by adding electrons (in green below)
Rh (s) + 2 H2O ---> RhO2 (s) + 4 H+ + 4 e-
Answer. The simplest way to balance half-reactions in basic solution is to balance in acid, then convert all H+ ions to water by adding hydroxide ions.(iii) (basic solution) RhO42- (aq) ---> Rh2O3 (s). Use same color code as established in part (i) to see how the rules work below:Above we balanced the half-reaction for an acid solution:
Rh (s) + 2 H2O ---> RhO2 (s) + 4 H+ + 4 e-
However, H+ is not dominant in basic solution, so let's add 4 hydroxide ions to each side of the equation:
Rh (s) + 2 H2O + 4 OH- ---> RhO2 (s) + 4 H+ + 4 OH- + 4 e-
Combine the hydrogen and hydroxide ions on the right-hand side to give water:
Rh (s) + 2 H2O + 4 OH- ---> RhO2 (s) + 4 H2O + 4 e-
Cancel water molecules on each side to get the final balanced half reaction in basic solution:
Rh (s) + 4 OH- ---> RhO2 (s) + 2 H2O + 4 e-
balanced in acid: 2 RhO42- + 10 H+ + 6 e----> Rh2O3 + 5 H2O(b) Give the letters (i, ii, or iii) of the half-reactions in part (a) that are oxidation half-reactions.to balance in base: add 10 OH- to each side of the equation, allow H+ to react with OH-, then cancel water molecules:
2 RhO42- + 10 H+ + 10 OH- + 6 e- ---> Rh2O3 + 5 H2O + 10 OH-
2 RhO42- + 10 H2O + 6 e- ---> Rh2O3 + 5 H2O + 10 OH-
Answer: 2 RhO42- + 5 H2O + 6 e- ---> Rh2O3 + 10 OH-
Answer: oxidation is defined as the loss of electrons; thus oxidation half-reactions have electrons on the right-hand side of the half-reaction. Reactions (i) and (ii) are oxidation half-reactions. Reaction (iii) is a reduction half-reaction since the reactant gains electrons (is reduced); thus electrons appear on the left-hand side.(c) Give the formulas of the reactants in part (a) that are oxidizing agents.Answer: oxidizing agents are the species that accept electrons. Thus RhO42- in part (iii) is an oxidizing agent. [In reactions (i) and (ii), Rh is a reducing agent because it provides electrons]
(a) (acid solution): RhO42- + Eu2+ ---> Rh2O3 + Eu3+Answer. (i) Write the two half reactions. One will be an oxidation half-reaction and the other will be a reduction half-reaction. (ii) Then multipy each by a number that will result in cancellation of electrons. (iii) Add the two halves. Cancel like terms on each side of the equation.(b) (basic solution): MnO4- + Fe(OH)2 ---> MnO2 + Fe(OH)3(2 RhO42- + 10 H+ + 6 e- ---> Rh2O3 + 5 H2O) x 1
(Eu2+ ---> Eu3+ + e- ) x 6
----------------------------------------------------------------
6 Eu2+ + 2 RhO42- + 10 H+ ---> Rh2O3 + 5 H2O+ 6 Eu3+
(MnO4- + 2 H2O + 3e- ---> MnO2 + 4 OH- ) x 1(Fe(OH)2 + OH- ---> Fe(OH)3 + e- ) x 3
----------------------------------------------------------------
MnO4- + 2 H2O + 3 Fe(OH)2 ---> MnO2 + 3 Fe(OH)3 + OH-
|
|
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM_2P32_Assign_8.html
Last revised: March 15 2001 by M. F. Richardson
© Brock University, 2001