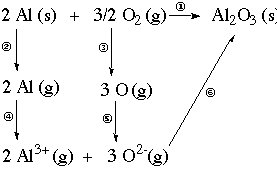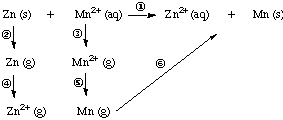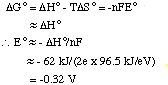CHEM 2P32 Hints for Assignment 9
The Elements
1. Hints. Lots of information look-up in this question.
-
Allotropes: Section 6.2
-
Conductors, semiconductors, and ferromagnetism: Section 6.6
-
Metal strength: Section 6.6. Hardness and softness are related to the numbers
of bonding electrons available in the metal
-
Catenation: Section 6.7
-
pi-bonding: Section 6.5. (pi-bonding is common when pi-bonds are stronger
than sigma bonds)
-
Metal-metal bonding (a form of catenation): Section 6.7
-
Liquids at or near room temperature: there are only 4 nonradioactive elements
that are liquids, 3 metals and one nonmetal. (Br2, Hg, Cs, Ga)
-
Gases at room temperature: 11 of them, all nonmetals.
-
Only known substance with 2 liquid forms: helium (p. 184)
2. Hints. See Section 6.4 for definitions of different kinds of
radii. As an approximation,
van der Waals radius and anionic radius are about the same.
They are the largest radii.
Covalent radius and single-bonded metallic radius are about the same,
and are about 60 pm smaller than the van der Waals/anionic radii.
Cation radius is the smallest, and is about 60 pm less than the covalent/single-bonded
metallic radii.
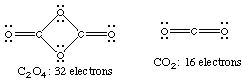
3. Example. Consider the reaction, 2 CO2 ---> C2O4.
C2O4 has a 4-membered ring with alternating carbons
and oxygens. Each carbon is also bonded to an additional oxygen.
(a) Draw Lewis structures for the product and the reactant.
Answer:
(b) Calculate the enthalpy change for this reaction from the
bond energies for products and reactants. The bond energy for C-O sigma
bonds is 358 kJ/mol; for C-O pi bonds it is 374 kJ/mol. [Note: sigma and
pi bond energies between two atoms of the same kind are in Table 6.5 in
Wulfsberg.] Answer:
The bond energy is the enthalpy change for breaking a bond in a molecule
with the reactants and products in the gas phase.
One mole of C2O4 has 6 C-O sigma bonds and 2 C-O
pi bonds. Thus 6 x (358 kJ/mol to break sigma bonds) + 2 x (374 kJ/mol
to break pi bonds) = 2896 kJ to break the bonds and form 2 moles of C atoms
and 4 moles of O atoms.
C2O4 ---> 2 C (g) + 4 O (g); Delta H
= +2896 kJ
Now let the C and O atoms combine to form two moles of CO2,
in which there are two sigma bonds and two pi bonds in each molecule. Thus
four moles of C-O sigma bonds and two moles of pi-bonds will be formed,
for a total energy gain of 4 x (-358 kJ/mol to form sigma bonds) + 4 x
(-374 kJ/mol to form pi bonds) = -2928 kJ
2 C (g) + 4 O (g) ---> 2 O=C=O; Delta H = -2928 kJ
Hess's Law states that when you add two (or more) reactions to give you
an overall reaction, the enthalpy change for the overall reaction is the
sum of the enthalpy changes of the individual reactions. Thus,
|
C2O4 ---> 2 C (g) + 4 O (g)
|
Delta H = +2896 kJ
|
|
2 C (g) + 4 O (g) ---> 2 O=C=O
|
Delta H = -2928 kJ
|
|
Overall: C2O4 ---> 2 O=C=O
|
Delta H = -2928 kJ + 2896 kJ = -32 kJ
|
(c) Which is more stable? Answer CO2 is more
stable because the enthalpy change for the overall rreaction is -32 kJ.
For problems 4, 5, and 6 you will need to make use of most or all of
the following tables:
Table 2.1: Hydration Energies
Table 6.1: Atomization Energies
Tables 6.6-6.8: 1st, 2nd, and 3rd Ionization Energies
Table 6.9: Electron Affinities
4. Example. The compound Al2O3 has a standard
enthalpy of formation equal to -1676 kJ/mol. Set up a thermochemical cycle
(i.e., use Hess's Law with atomization energies, ionization energies, etc.)
and calculate the lattice energy of Al2O3.
Solution. A thermochemical cycle may be shown as a diagram,
or as a set of equations (see following the figure). In the figure, the
overall reaction is represented on the top line (step 1). This reaction
defines the enthalpy of formation for Al2O3.
A thermochemical cycle shows that reactants can form a product
in a single step (step 1 below), or in a series of steps (Steps 2, 3, 4,
5, and 6). Whichever way the reaction occurs, the enthalpy change is the
same. This is what Hess's Law says: the enthalpy change associated
with a reaction is the same whether the reaction proceeds in one step or
many steps.
|
Hess's Law: If a reaction
can be written as the sum of two or more constituent reactions, the enthalpy
change for the overall reaction is the sum of the enthalpy changes for
the constituent reactions.
|
Thus the enthalpy associated with step 1 is the sum of the enthalpy
changes of steps 2, 3, 4, 5, and 6.
-
In steps 2 and 3, we imagine atomizing the elements (atomization energies).
Table
6.1
-
In steps 4 and 5, we let them lose or gain electrons to form the ions needed
in the final compound (ionization energies and electron affinities). Tables
6.6 - 6.9
-
In step 6 we let the gaseous ions combine to form the crystalline compound
(lattice energy).
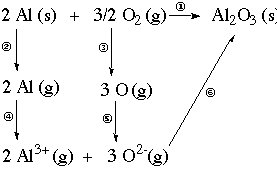
Chemical equations and their associated enthalpies are shown
below. Keep in mind that the nth electron affinity is the ionization
energy of the -n ion:
|
Definition
|
Equation
|
Delta H, kJ/mol
|
|
1st electron affinity of O
|
O- (g) ---> O (g) + e-
|
141
|
|
2nd electron affinity of O
|
O2- (g) ---> O- (g) + e-
|
-780
|
(Note that the positive sign for the first electron affinity means
that the O- ion is stable with respect to losing an electron.
However, the O2- ion is NOT stable in the gas phase.
The oxide ion only exists in ionic compounds where the cation attraction
to an O2- ion is high enough to outweigh the unfavourable electron
affinity of forming the ion. Coulomb's law at work again!)
|
Step
|
Equation
|
Delta H, kJ
|
|
2: Atomization energy of Al
|
2 Al (s) ---> 2 Al (g)
|
2 x 326 kJ/mol = 652 kJ
|
|
3: Atomization energy of O
|
3/2 O2 (g) ---> 3 O (g)
|
3 x 249 kJ/mol = 747 kJ
|
|
4. Ionization of Al: 1st IE
|
2 Al (g) ---> 2 Al+ (g) + 2 e-
|
2 x 578 kJ/mol = 1156 kJ
|
|
2nd IE
|
2 Al+ (g) ---> 2 Al2+ (g) + 2 e-
|
2 x 1817 kJ/mol = 3634 kJ
|
|
3rd IE
|
2 Al2+ (g) ---> 2 Al3+ (g) + 2 e-
|
2 x 2745 kJ/mol = 5490 kJ
|
|
5. Ionization of O: 1st EA
|
3 O (g) + 3 e- ---> 3 O- (g)
|
3 x -141 kJ/mol = -423 kJ
|
|
2nd EA
|
3 O- (g) + 3 e- ---> 3 O2- (g)
|
3 x 780 kJ/mol = 2340 kJ
|
|
6. Formation of Al2O3 (s) from its ions (the
lattice energy)
|
2 Al3+ (g) + 3 O2- (g) ---> Al2O3
(s)
|
Lattice energy
|
Sum of Steps 2-6
= Step 1
|
2 Al (s) + 3/2 O2 (g) ---Al2O3(s)
|
= 13596 kJ + Lattice Energy
= -1676 kJ (standard enthalpy of formation of Al2O3
(s))
|
Thus the lattice energy is -1676 kJ - 13596 kJ = -15272 kJ
5. Example. Consider the reaction Zn (s) + Mn2+ (aq)
---> Zn2+ (aq) + Mn (s).
(a) Set up a thermochemical cycle and calculate the enthalpy
of reaction.
(b) Ignoring entropy effects, calculate Eo for this reaction.
(c) Does this reaction go as written to produce products?
Solution Part (a). Here is the thermochemical cycle:
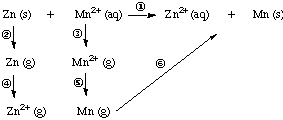
|
Step
|
Equation
|
Delta H, kJ
|
|
2 (atomization energy of Zn)
|
Zn (s) ---> Zn (g)
|
131 kJ
|
|
3 (dehydration of Mn2+ (aq))
|
Mn2+ (aq) ---> Mn2+ (g)
|
1845 kJ
|
|
4 (ionization of zinc) 1st IE
|
Zn (g) ---> Zn+ (g) + e-
|
906 kJ
|
|
2nd IE
|
Zn+ (g) ---> Zn2+ (g) + e-
|
1733 kJ
|
|
5 (reduction of Mn2+) -2nd IE
|
Mn2+ (g) + e- ---> Mn+ (g)
|
-1509 kJ
|
|
-1st IE
|
Mn+ (g) + e- ---> Mn (g)
|
-717 kJ
|
|
6 (hydration of Zn2+ (g))
|
Zn2+ (g) ---> Zn2+ (aq)
|
-2044 kJ
|
|
6 (condensation of Mn atoms)
|
Mn (g) ---> Mn (s)
|
-283 kJ
|
|
Sum of steps 2-6 = overall reaction (step 1):
|
Zn(s) + Mn2+ (aq) ---> Zn2+ (aq) + Mn (s)
|
ANSWER: 131+1845+906+1733-1509-717-2044-283
= 62 kJ
|
(b) Ignoring entropy effects means that we will assume that the
entropy change is zero. In this reaction, two electrons are involved as
Zn is oxidized to the +2 ion and the Mn2+ ion is reduced to
manganese. Thus n = 2 in the equation below.
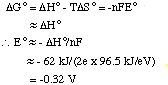
(c) The reaction isn't expected to go to any appreciable extent
since the enthalpy change is positive (leading to a negative value of Eo
for the reaction.)
6. Hints. This question works the same way as the previous two
problems. You'll be using atomization energies, ionization energies (or
their reverse) for metals and hydrogen, electron affinities for halogens,
and hydration energies for all ions involved. Just proceed in an orderly
manner as we have done in the above examples.
Back
to Chem 2P32 Home Page
Back
to Assignment Schedule
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM_2P32_Assign_9.html
Last revised: March 27 2001 by M. F. Richardson © Brock University,
2001