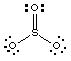
1. (15 marks) Consider the oxides of the following ions: Co2+, S6+, Ti4+, Cs+, Zn2+. Answer the following questions. Note that a particular oxide may be used more than once or may not be used at all in answer to these questions.
(a) Give the formula of the oxide most likely to give an acidic solution when dissolved in water. Write an equation for the reaction.
SO3 - has the most acidic cation. It is the acid anhydride for sulfuric acid.(b) Give the formula of the oxide most likely to be colored. Why is it colored?SO3 + H2O ---> H2SO4
CoO - Co2+ is a d7 cation, so the transitions within the split d-orbitals will make it colored.(c) Give the formula of the oxide most likely to be amphoteric. Write an equation for its reaction with an acid; with a base.
ZnO (or CoO). Amphoterism is found in oxides of +2 ions such as Be2+, Zn2+, and Sn2+, or small +3 ions such as Al3+(d) Give the formula of the oxide most likely to dissolve in water to give a basic solution. Write an equation for the reaction.ZnO + 2 HCl ---> ZnCl2 + H2O
ZnO + H2O + 2 NaOH ---> Na2[Zn(OH)4]
Cs2O. Cs+ has no acid character, and the oxide ion is a strong base. The Group 1A oxides are anhydrides to the common strong bases such as KOH and NaOH.(e) Give the formula of the oxide most likely to occur as gaseous molecules. Draw its Lewis structure.Cs2O + H2O ---> 2 CsOH
Molecular oxides are generally found in compounds between and a nonmetal, whereas metal oxides exhibit ionic bonding and are network structures. SO3 is a gas.2. (13 marks) (a) (6 marks) Explain, with equations and/or drawings, the difference between hydration and hydrolysis of an anion such as PO43-.
The oxygens on phosphate, each of which carries an average charge of -3/4 (total charge of -3 spread over 4 oxygens), are hydrogen-bond acceptors from water molecules. The size of phosphate is large enough (about 220 pm in radius) to accomodate a dozen water molecules about itself. A schematic drawing of a hydrated phosphate ion is shown below.(b) (7 marks) (i) What is meant by the acidity of a metal cation? Explain with a chemical equation.
The phosphate ion attracts the hydrogen from a water molecule to produce the hydrogen phosphate ion and hydroxide, a process known as hydrolysis.
PO43-(aq) + H2O ---> HPO42- + OH-
Metal cations behave as Bronsted acids in water. For example, solutions of aluminum chloride are acidic because the hydrated aluminum ion exerts an inductive effect on the O-H bonds, resulting in hydrolysis and production of H3O+ ions.(ii) Arrange the following cations in order of their acidity: Mg2+, Al3+, K+, Zn2+, C4+. Briefly explain how you arrived at the order.Al(H2O)63+ + H2O ---> Al(H2O)5(OH)2+ + H3O+
Cation acidity is determined primarily by the charge Z and size (radius) r. There is an approximate linear relationship between the pKa of the hydrated ion and Z2/r of the cation. The electronegativity of the cation also affects the acidity, but to a lesser extent than the charge, and only if the electronegativity is greater than about 1.8.3. (12 marks) (a) (6 marks) Which compound would you expect to have the highest melting point: NaF, CaF2, or CaCl2? Which the lowest? Explain how you arrived at your answers.In order of Z2/r, with Zn2+ being rated more acidic than Mg2+ because of its higher electronegativity: C4+ > Al3+ > Zn2+ > Mg2+ > K+.
When an ionic compound melts, the forces that must be overcome are the ionic bonds between cations and anions. The energy of attraction between them is proportional to the product of the charges on the ions divided by the contact distance in the crystal lattice:(b) Draw diagrams of the NaCl and CsCl unit cells, and show/describe the coordination of the Na+ and Cs+ ions. Briefly explain why the two structures aren't the same.Eionic proportional to (Z+)(Z- )/d
CaF2 will melt higher than CaCl2 because the Ca2+-F- distance is shorter than the Ca2+-Cl - distance. Both compounds will melt higher than NaF because of the higher charge on the calcium ion compared to the sodium ion.
In NaCl, the sodium ions and the chloride ions are both octahedral 6-coordinate. The unit cell has chloride ions at all the corners and in the center of every face. The sodium ions lie in the center of each of the 12 edges, and in the center of the cell.4. (12 marks) (a) (8 marks) Explain what is meant by isomorphous replacement and when it occurs. Use the anorthite-albite series as an example. Albite is NaAlSi3O8, anorthite is CaAl2Si2O8. Why is it common in network structures but not in molecular compounds?In CsCl, the chloride ions form a simple cubic packing at the corners of the cell, and there is a cesium ion in the center. The cesium ions and the chloride ions are both cubic 8-coordinate.
The CsCl structure is different from the NaCl structure because cesium is so much larger, and can accomodate 8 chlorides about itself.
Isomorphous replacement occurs when ions of a similar size and the same charge replace each other in a crystalline material without changing the structure of the crystal. Albite and anorthite form a continuous series in which gradually increasing amounts of [Na+ + Si4+] are replaced by [Ca2+ + Al3+](b) (4 marks) Two precious stones, emerald and ruby, contain the Cr3+ ion. Emerald is the green form of beryl, Be3Al2[Si6O18]; Ruby is the red form of corundum, Al2O3. In both cases, a small amount of Al(III) has been replaced by Cr(III). Use crystal field theory and explain why the colors are different.One Si4+ in albite is replaced by Al3+, which is about 20% larger than Si4+. However, the charges aren't balanced now, so Na+ must be replaced by Ca2+, which is about the same size as Na+. Now the charges [Ca2+ + Al3+] are the same as the charges [Na+ + Si4+] in the original compound. This process can continue until every Si4+ and Na+ in albite are replaced by Al3+ and Ca2+.
It doesn't occur in molecular compounds because, e.g., replacing 10% of Mn in Mn2O7 by Tc gives a mixture that contains Tc2O7, Mn2O7, and TcMnO7. These three compounds could be separated by chromatography or some other method, unlike solid structures where the individual compounds that comprise the "end members" cannot be separated.
Cr3+ is a d3 ion. In octahedral coordination, the electron configuration is t2g3eg0. The colors are different because the crystal field splitting energy is different.5. (16 marks) (a) (10 marks) Explain how and why the basicity of an oxo anion depends on its charge, the number of oxo groups present, and the electronegativity of the central atom.
The low splitting energy for Cr3+ in beryl results in the red part (low energy) of the spectrum being absorbed, so the transmitted color appears green. In corundum, the splitting energy is higher so a higher-energy part of the visible spectrum is absorbed, so the low-energy part of the spectrum is transmitted (red).
Refer to the diagram in Question 2a. Basicity of an oxo anion increases as the negative charge on the ion increases. The greater the negative charge on the oxo ion (e.g. in the series ClO4-, SO42-, PO43-) , the more the proton in the water molecule will be attracted to the ion, leading to hydrolysis. Thus ClO4- is the least basic and PO43- the most basic ion.(b) (6 marks) Use your answer to part (a) above to predict the relative basicities of the following three phosphate ions. Show your reasoning.Basicity decreases with increasing number of oxo groups if the charge is fixed. The more oxo groups there are for a given charge, the more the negative charge can be spread around. Comparing two ions with the same charge, but different numbers of oxo groups [ClO- and ClO4- ], there is only one oxo group in ClO- so it carries the full negative charge (O being more electronegative than Cl). However, in ClO4- there are four oxo groups over which to spread the charge, so each has a -1/4 charge and will be less able to attract a proton from a water molecule. [Can also argue that the more oxo groups, the higher the oxidation number of the central atom and thus the greater the inductive effect: as the central atom attracts more electrons to itself from the oxygen, there is less electron density on the oxygen to bond to a proton so the base will get weaker with increasing oxidation number of the central atom.]
The more electronegative the central atom, the weaker the base. This is because a more electronegative central atom a greater its ability to attract electron density from the oxygens, leaving less electron density to bond to protons. Thus ClO- is a stronger base than IO-.
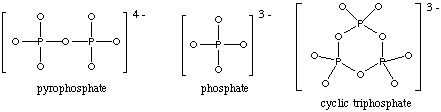
Method A. Charge distribution per oxo group.6. (12 marks) Answer the following questions by giving one or more of the following ions as the correct response. If no ion is a satisfactory answer to the question, write "none." Some ions may be used more than once, some may not be used at all.
ion # oxo groups charge per oxo group pyrophosphate 6 -4/6 = -0.667 phosphate 4 -3/4 = -0.75 cyclic triphosphate 6 -3/6 = -0.50 Based on the charge distribution per oxo group, phosphate is the most basic and cyclic triphosphate the least. NOTE: oxo groups are oxygens bonded to only one other atom.
Method B. Cancelling 2 oxo groups with one negative charge
ion # oxo groups charge after cancellation pyrophosphate 6 -4 -1 spread over 2 P = -1/2 per P phosphate 4 -3 -1 for P cyclic triphosphate 6 -3 0 Same results as Method A, you just have to remember that the negative charges or excess oxo groups have to be spread over all the phosphorus atoms.
Ions: Cs+, Bu4N+, Mg2+, Fe3+, PO43-, MnO4-, SO42-(i) Which cation will form the largest hydrated ion? Fe3+
(ii) Which cation(s) will be electrostatic structure makers? Fe3+, Mg2+
(iii) Which cation(s) will disrupt the icebergs in liquid water? Cs+
(iv) Which anion is an electrostatic structure breaker? MnO4-
(v) Which ion is a hydrophobic structure maker? Bu4N+
(vi) Which cation(s) are likely to give insoluble salts with the perchlorate ion? Cs+, possibly Bu4N+
(vii) Which cation(s) are likely to give insoluble salts with the phosphate ion? Fe3+, Mg2+
(viii) Write the formula of any salt containing just these ions that will precipitate for reasons connected with an entropy change. FePO4 or Mg3(PO4)2
(ix) Write the formula of any salt containing just these ions that will
precipitate for reasons connected with an enthalpy change.
CsMnO4
7. (10 marks). Draw and briefly discuss the structures of typical silicates and aluminosilicates, showing how complex silicates are built up from the simple orthosilicate anion. You may wish to use the following silicates as examples in your answer: Na[AlSi3O8] (albite), CaMg[SiO3]2 (diopside), (Mg,Fe)2[SiO4] (olivine), Be3Al2[Si6O18] (beryl), and Mg3[Si4O10](OH)2 (talc).
This is well-covered in your text. Just make sure that you calculate the O:Si or O"(Si+Al) ratio in the silicate or aluminosilicate anions (enclosed in square brackets). Practice drawing the chains and sheets!8. (10 marks). (a) (5 marks) Given the following data:
Enthalpy of hydration for the sulfate ion = -1115 kJ/mol
Enthalpy of hydration for the barium ion = -1304 kJ/mol
Lattice energy of BaSO4 = -2423 kJ/mol
(i) What is the definition of the lattice energy?
Energy released when gaseous cations and anions come together to form 1 mole of the crystalline salt(ii) Write the chemical equation for which the enthalpy change is -2423 kJ/mol
Ba2+ (g) + SO42- (g) ---> BaSO4 (s)(iii) Write the chemical equation for which the enthalpy change is -1115 kJ/mol.
SO42- (g) ---> SO42- (aq)(b) (5 marks) Calculate the enthalpy of precipitation for BaSO4 from the data in part (a). Show your work clearly, including a chemical equation for which the enthalpy change is the enthalpy of precipitation.
Precipitation of BaSO4: Ba2+ (aq) + SO42- (aq) ---> BaSO4 (s). The enthalpy of precipitation is the enthalpy change for this reaction, so put the reactions in an order that adds to give this equation.
Reaction Enthalpy change SO42- (aq) ---> SO42- (g) 1115 kJ/mol x 1 mol = 1115 kJ Ba2+ (aq) ---> Ba2+ (g) 1304 kJ/mol x 1 mol = 1304 kJ Ba2+ (g) + SO42- (g) ---> BaSO4 (s)
-2423 kJ/mol x 1 mol = -2423 kJ
Ba2+ (aq) + SO42- (aq) ---> BaSO4 (s) Answer: -4 kJ for the enthalpy of precipitation
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/Midterm_Exam_2_2001.html
Created April 6, 2001 by M. F. Richardson
© Brock University, 2001