 Tomas Hudlicky
Tomas Hudlicky 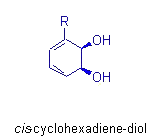 Our group is engaged in a variety of projects ranging from total synthesis
to investigations of new reactions and the design of enzyme inhibitors.
In total synthesis, we work on implementing reliable and efficient routes
to target molecules. Our ventures are exact and logical pursuits, yet serendipity,
intuition, and art all form an integral part of designing a total synthesis.
Our group is engaged in a variety of projects ranging from total synthesis
to investigations of new reactions and the design of enzyme inhibitors.
In total synthesis, we work on implementing reliable and efficient routes
to target molecules. Our ventures are exact and logical pursuits, yet serendipity,
intuition, and art all form an integral part of designing a total synthesis.
 Recently we have exploited the biooxidation of aromatic compounds in an
exhaustive approach to the synthetic design of carbohydrates and their
derivatives. Our guiding principles are symmetry, simplicity, and precise
order of operations so that any derivative or stereoisomer with a sugar
backbone can be constructed. These products are tested for glycosidase
inhibition, a process important in viral expression. In addition, carbocyclic
sugars can act as cell messengers, and their availability through synthesis
allows greater understanding of cellular communication.
Recently we have exploited the biooxidation of aromatic compounds in an
exhaustive approach to the synthetic design of carbohydrates and their
derivatives. Our guiding principles are symmetry, simplicity, and precise
order of operations so that any derivative or stereoisomer with a sugar
backbone can be constructed. These products are tested for glycosidase
inhibition, a process important in viral expression. In addition, carbocyclic
sugars can act as cell messengers, and their availability through synthesis
allows greater understanding of cellular communication.
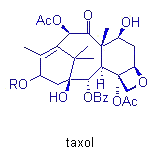
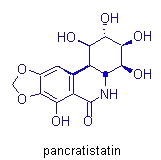 Morphine, pancratistatin, and taxol are other important molecules in which
our group has invested much synthetic effort. Their total synthesis permits
the investigation of new reactions and mechanistic pathways, which can
then be applied in subsequent syntheses.
Morphine, pancratistatin, and taxol are other important molecules in which
our group has invested much synthetic effort. Their total synthesis permits
the investigation of new reactions and mechanistic pathways, which can
then be applied in subsequent syntheses.
 Finally we are devoting some effort to studies in the mechanism of prokaryotic
oxygenase enzymes. Our ultimate goal is the design of a synthetic enzyme
mimic that can be used as a chiral reagent for aromatic cis-hydroxylation.
Finally we are devoting some effort to studies in the mechanism of prokaryotic
oxygenase enzymes. Our ultimate goal is the design of a synthetic enzyme
mimic that can be used as a chiral reagent for aromatic cis-hydroxylation.
Aldrichimica
Acta Volume 32, Number 2, 1999
Enzymatic
Dihydroxylation of Aromatics in Enantioselective Synthesis: Expanding Asymmetric
Methodology