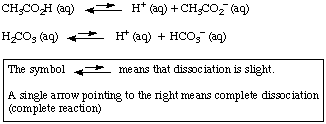Lecture 14
Reactions in Aqueous Solutions: Part 2
A. Acids and Bases: Overview
|
Compound Type
|
Characteristic Behaviour
|
Chemical Characteristics
|
|
Acids
|
Taste sour, react with many metals to produce
hydrogen gas. React with bases to produce salts in a
neutralization reaction.
|
Produce H+ (hydrogen ion or
proton) in solution
|
|
Bases
|
Taste bitter, feel slippery, don't react with
most metals, but do form insoluble hydroxides with most
metal cations. React with acids to produce salts in a
neutralization reaction.
|
Produce OH- (hydroxide ion) in
solution
|
B. Strong and Weak Acids; Monoprotic and Polyprotic Acids
Strong acids are acids that dissociate
completely when they dissolve in water. Among the strong acids are
hydrochloric and nitric acid:
HCl (aq) ---> H+ (aq) + Cl-
(aq)
HNO3 (aq) ---> H+ (aq) +
NO3- (aq)
Weak acids are acids that dissociate incompletely upon
dissolution in water. Among the weak acids are acetic acid and
carbonic acid:
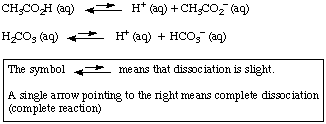
Carbonic acid undergoes dissociation in two stages. The hydrogen
carbonate ion produced in the first stage further dissociates:
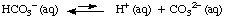
Monoprotic acids are acids that provide only 1 mole of
H+ per mole of acid. HCl and HNO3 are obviously
monoprotic. CH3CO2H is also monoprotic. Only
one of its four hydrogens is ionizable.
Polyprotic acids are acids that can provide more than 1
mole of H+ per mole of acid. Carbonic acid
(H2CO3) is one such acid; sulfuric acid
(H2SO4) is another. Both carbonic and sulfuric
acids contain 2 ionizable hydrogens. Phosphoric acid
(H3PO4) can ionize to produce 3 H+
per mole of acid.
C. Strong and Weak Bases
Strong bases are generally metal hydroxides
such as NaOH and Ba(OH)2. They dissociate completely into
their ions in water:
NaOH ---> Na+ (aq) + OH- (aq)
Ba(OH)2 ---> Ba2+ (aq) + 2 OH-
(aq)
A distinction can be made between strong soluble bases and
strong insoluble bases. Not many metal hydroxides are soluble;
the ones that are comprise the strong soluble bases. Hydroxides that
are only slightly soluble in water (such as calcium hydroxide or
iron(III) hydroxide) are strong bases, because whatever amount does
dissolve dissociates completely into the ions.
Weak bases include ammonia (NH3) and organic
amines such as methylamine (CH3NH2). They do
not contain the hydroxide ion, but they produce it when they dissolve
in water because they react with water:
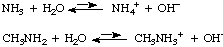
For a listing of common acids and bases see Table 5.2 in Kotz and
Treichel.
D. Reactions of Acids with Strong Bases (Metal Hydroxides)
The fact that acids react with bases to produce salts
is highly important in chemistry, biology, and the earth and
environmental sciences.
If the base is a metal hydroxide (soluble or insoluble), water is
the other product of the reaction. Thus, an acid reacts with a
metal hydroxide to produce a salt and water. Some examples:
(a) reaction of a strong acid with a strong soluble
base:
2 HCl (aq) + Ba(OH)2 (aq) ---> BaCl2 (aq)
+ 2 H2O (l)
(b) reaction of a weak acid with a strong soluble base:
CH3CO2H (aq) + NaOH (aq) --->
NaCH3CO2 (aq) + H2O (l)
(c) reaction of a strong acid with a strong insoluble base:
3 H2SO4 (aq) + 2 Al(OH)3 (s)
---> Al2(SO4)3 (aq) + 6
H2O (l)
The
net
ionic equations (Lecture 13) for these 3 reactions are:
(a) H+ (aq) + OH- (aq) --->
H2O (l)
(b) CH3CO2H (aq) + OH- (aq)
---> CH3CO2- (aq) +
H2O (l)
(c) 3 H+ (aq) + Al(OH)3 (s) --->
Al3+ (aq) + 3 H2O (l)
If the acid is polyprotic, the neutralization proceeds in stages.
The final product depends on the amount of base used in the reaction.
Here are the overall equations for the reaction of phosphoric acid (a
weak triprotic acid) with different amounts of potassium hydroxide:
(d) H3PO4 (aq) + KOH (aq)
---> KH2PO4 (aq) + H2O (l)
(e) H3PO4 (aq) + 2 KOH (aq) --->
K2HPO4 (aq) + 2 H2O (l)
(f) H3PO4 (aq) + 3 KOH (aq) --->
K3PO4 (aq) + 3 H2O (l)
The net ionic equations for these 3 reactions are:
(d) H3PO4 (aq) + OH- (aq)
---> H2PO4- (aq) + H2O
(l)
(e) H3PO4 (aq) + 2 OH- (aq) --->
HPO42- (aq) + 2 H2O (l)
(f) H3PO4 (aq) + 3 OH- (aq) --->
PO43- (aq) + 3 H2O (l)
E. Reactions of Acids with Weak Bases
Weak bases do not contain hydroxide in their formulas.
When they react with acids, they do so by removing a proton (hydrogen
ion) from the acid so as to become the cation. A salt is produced,
but no water. For example, when aqueous nitric acid reacts with
aqueous ammonia, the salt ammonium nitrate is produced. The overall
reaction is:
HNO3 (aq) + NH3 (aq) --->
NH4NO3 (aq)
The net ionic equation for this reaction is
H+ (aq) + NH3 (aq) --->
NH4+ (aq)
Similarly, if carbonic acid reacts with an equimolar amount of
ammonia, ammonium hydrogen carbonate is produced:
H2CO3 (aq) + NH3 (aq)
---> NH4HCO3 (aq)
The net ionic equation for this reaction is
H2CO3 (aq) + NH3 (aq)
---> NH4+ +
HCO3+ (aq)
F. Acidic and Basic Oxides
Oxides that react with water to produce acids are
called acidic oxides or acid anhydrides. Carbon dioxide
and sulfur trioxide are two such compounds. Carbon dioxide reacts
with water to produce carbonic acid:
CO2 (g) + H2O (l) --->
H2CO3 (aq)
Sulfur trioxide reacts with water to produce sulfuric acid:
SO3 (g) + H2O (l) --->
H2SO4 (aq)
Most acidic oxides are oxides of nonmetals, or of metals that are
in very high oxidation states. An example of an acidic metal oxide is
CrO3, which reacts with water to produce chromic acid,
H2CrO4.
Oxides that react with water to produce bases are called basic
oxides or base anhydrides. Sodium oxide reacts with water
to produce the strong soluble base sodium hydroxide:
Na2O (s) + H2O (l) ---> NaOH
(aq)
Calcium oxide ("lime") reacts with water to produce the insoluble
base calcium hydroxide ("slaked lime"):
CaO (s) + H2O (l) --->
Ca(OH)2 (s)
G. Gas-forming Reactions
Important gases that may be evolved in chemical
reactions are shown in the Table below.
|
Gas
|
Produced in this kind of reaction:
|
|
Hydrogen (H2)
|
Metal + acid
Example: Mg (s) + 2 HCl (aq) ---> MgCl2
(aq) + H2 (g)
|
|
Carbon dioxide (CO2)
|
Metal carbonate or bicarbonate + acid
Example: Na2CO3 (aq) + 2
HNO3 (aq) ---> 2 NaNO3(aq) +
H2O (l) + CO2 (g)
|
|
Hydrogen sulfide (H2S)
|
Metal sulfide + acid
Example: FeS (s) + H2SO4 (aq)
---> FeSO4 (aq) + H2S (g)
|
|
Sulfur dioxide (SO2)
|
Metal sulfite or hydrogen sulfite + acid
Example: NaHSO3 + H2SO4
(aq) ---> SO2 (g) + NaHSO4 (aq) +
H2O (l)
|
|
Ammonia (NH3)
|
Ammonium salt + strong base
Example: NH4NO3 (aq) + NaOH (aq)
---> NaNO3 (aq) + NH3 (g) +
H2O (l)
|
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_14.html
Created September 11, 2000 by M. F. Richardson
© Brock University, 2000