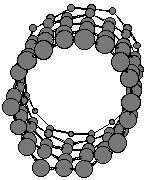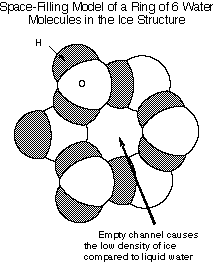Lecture 2: Structure of Matter; Physical & Chemical Change
1. Elements and atoms
Elements are made of one type of atom, e.g. diamond consists
only of carbon atoms; aluminum foil contains only aluminum atoms.
The periodic table arranges elements in order of their atomic number
(number of protons in the nucleus), so that elements with similar properties
are arranged in columns.
2. Compounds and mixtures
Compound - contains 2 or more elements with a fixed ratio of
the number of atoms. Every compound has its own characteristic physical
and chemical properties.
More than one compound may be formed by the same two elements, for example
water and hydrogen peroxide. However, the atomic ratios are different.
H2O is water; the water molecule contains two hydrogen
atoms and one oxygen atom
H2O2 is hydrogen peroxide; each molecule contains
two hydrogen atoms and two oxygen atoms
A solution of salt in water is not a compound, because the proportions
of salt and water may be varied indefinitely. Such a solution is a mixture.
Mixtures
may be homogeneous or
heterogeneous (see
Lecture
1).
3. Classifications of compounds: molecular and network structures
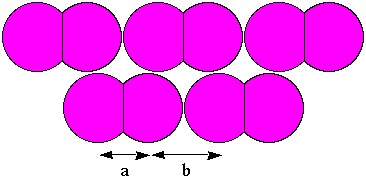
Molecular structures: at the microscopic level, individual
molecules can be distinguished. Distances between the atoms that make up
the molecule are small compared to the distances between molecules. Iodine
is an example of a compound with distinguishable diatomic molecules.
Distance "a" is the distance between the centers of I atoms in the same
molecule. Distance "b" is the distance between I atoms in two adjacent
molecules.
Network structures: at the microscopic level, there are no distinguishable
molecules. The atoms or ions form a regular lattice. Salt (sodium chloride,
NaCl) is an example of an ionic network structure.
In the picture below, you can see how the sodium ions (green) and chloride
ions (red) alternate regularly so that there is no distinguishable "molecule"
of NaCl.

4. Physical and chemical changes
A physical change does not involve the creation or destruction
of a chemical compound. Examples of physical changes:
melting of ice to form liquid water
dissolving sugar in water to form a solution
breaking a stick of wood into smaller pieces
A chemical change DOES involve the creation of new chemical substances.
Examples of chemical changes:
burning gasoline in your car's engine to form carbon dioxide
and water
the reaction of hydrogen and oxygen to form water
the electrolysis of NaCl to form metallic sodium and chlorine gas
5. Representations of a molecule
a. By a formula (symbolic representation). Methane is the chief
component of natural gas. It is a compound of carbon and hydrogen, and
its formula is CH4.
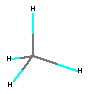
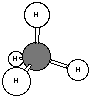
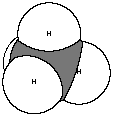
b. By a drawing to show how the atoms are bonded.
c. By models (or pictures of models, as shown here) to represent not
only the correct bonding, but also the shape of the molecule. For instance,
methane is not a flat molecule, it has a three-dimensional shape called
tetrahedral
. Models may be stick models, ball-and-stick models, or space-filling
models (shown below). Ball-and-stick is most common in chemistry unless
there is a specific reason to use one of the other two.
6. Application: Nanotubes (National Post, Sept. 12, 2000, p. A17)
Almost everyone knows that carbon exists in more than a single
form. Diamond and graphite are two well-known forms of carbon (both are
network structures), and many students know about "buckyballs" that are
spherical C60 molecules. However, in the last few years carbon
has also been found to form tubular structures based on graphite. These
tubes are called "nanotubes" because their diameter is on the order of
a few nanometers (1 nm = 1 x 10-9 m). They have exciting properties
and are the object of intensive research.
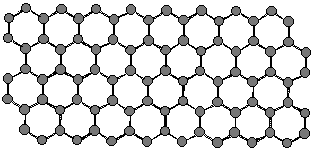
Let's look at how nanotubes are formed from graphite. Graphite consists
of layers of carbon atoms with each carbon joined to three others to make
a planar network of hexagonal rings. Below is a ball-and-stick model for
part of the graphite structure.
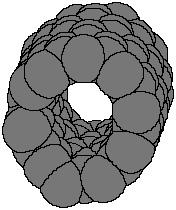
Now if we imagine that we take this planar structure and hook together
the carbons on the right- and left-hand sides, we get a tube, shown both
as ball-and-stick and as space-filling models. The "hole" in the center
of the tube is clearly visible. The hole size varies with the number of
carbons in the graphite sheet. The holes can be filled with other atoms
or molecules, which makes possible endless ideas for how nanotubes might
be used in the future.
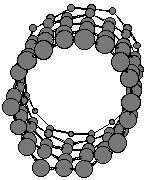
7. Application: Ice, liquid water, and the density of H2O.
Let's look at the density of water as a function of temperature:
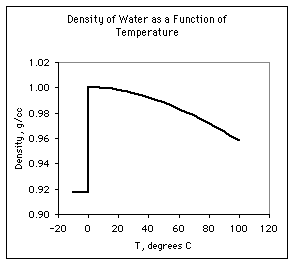
Above zero degrees C, we notice that the density of water decreases
steadily. We interpret this in terms of the kinetic-molecular theory, that
molecules move more rapidly with increasing temperature, thus increasing
the distance between them and the volume that the liquid water occupies.
Since density is the mass divided by the volume, the density falls.
But how about ice? The molecules in ice aren't moving very much, so
why is it that a given mass of ice occupies more volume than the
same mass of liquid water? (I.e., ice has a lower density than liquid water).
To find the answer we need to look at the structures of ice and consider
what happens when ice melts.
Ice is made up of water molecules linked by hydrogen bonds (weak interactions
between water molecules) to form a very open structure made of linked rings
containing 6 water molecules:
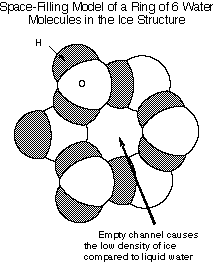
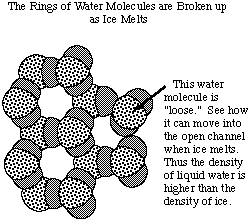
However, at zero degrees C, some of the water molecules move enough
to start breaking up these 6-membered rings. Then water molecules can twist
and turn to start filling the "empty" space that is found in the rigid
ice structure. Thus the volume occupied by the liquid water is less than
the volume occupied by solid ice, and the density (=mass/volume) is higher
for liquid water than for ice.
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_2.html
Created September 12, 2000 by M. F. Richardson
© Brock University, 2000