Lecture 22
Calorimetry
A. Types of Calorimeters
Calorimeters are devices for measuring the amount of
heat generated by a chemical reaction or other process (such as a
phase change). There are two main types:
- The bomb calorimeter that measures heat changes at
constant volume. Since the heat change is measured at constant
volume, it is the internal energy change that is being
determined.
- Calorimeters, such as the ice calorimeter and coffee-cup
calorimeter, that measure heat changes at constant pressure. Since
the heat change is measured at constant pressure, it is the
enthalpy change that is being determined.
B. Bomb Calorimeter
Figure 6.17 in Kotz and Treichel shows a schematic
view of a bomb calorimeter. It is called a "bomb" because, when a
combustible sample is burned in pure oxygen, the reaction is so rapid
that it is essentially an explosion.
The components of a bomb calorimeter are:
- a steel sample chamber which contains the combustible sample
and the oxygen
- a container of water (insulated) to absorb the heat given off
by the combustion
- a thermometer to measure the temperature change of the water
The experimental procedure is simple:
- weigh the sample and place it in the combustion chamber
- weigh the calorimeter
- add water to the calorimeter and weigh again to get the weight
of the water
- assemble the apparatus and measure the beginning temperature
of the water
- ignite the sample by means of a spark
- measure the temperature of the water when thermal equilibrium
has been re-established.
Now, we know the mass of the calorimeter, the mass of the water,
the mass of the sample, and the temperature change that took place
when the sample burned. From these data, the specific heat of water,
and the heat capacity of the calorimeter, we can calculate the heat
of combustion of the sample.
Heat evolved by the reaction = heat gained by the
calorimeter and the water
C. Example: Determination of the Heat of Combustion of
Caffeine
See sample
question
9 in Assignment 8.
D. The Ice Calorimeter
Experiment 7 in your lab involves the use of the an
"ice calorimeter" to measure the heat produced when magnesium metal
reacts with an acid. The heat of reaction is measured by determining
how much ice melts.
qreaction + qice = 0
qice = (mass of ice melted) x (heat of fusion
of ice (333 J/g))
The mass of ice melted is determined indirectly in this
experiment: it is based on the change in volume that takes place when
ice melts. Ice has a density of 0.917 g/mL at 0o C,
whereas water has a density of 1.000 g/mL at 0o C. Thus
equal masses of ice and water have quite different volumes:
Volume occupied by 100.0 g ice: 100.0 g x 1 mL / 0.917
g = 109.0 mL
Volume occupied by 100.0 g water = 100.0 mL
The large volume change can be used to determine the mass of ice
melted, as follows. When a given mass of ice melts to form the same
mass of water, the volume change is given by
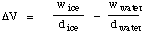
Now since wice = wwater = w,
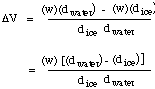
Solving for w, the weight (mass) of ice melted by the reaction, we
obtain
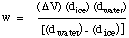
Thus, the mass of ice that melted is easily determined from the
volume change that you will measure in the lab, along with the
densities of water and of ice at 0o C. The calculation for
the enthalpy of reaction proceeds as in Question 8 on
Assignment
8.
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_22.html
Created October 31, 2000 by M. F. Richardson
© Brock University, 2000