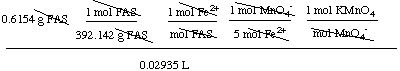Hints for Assignment 6 1998-1999
1. Hint. See examples on p. 214, Kotz and Treichel
2. Example. You have a solution of 0.2009 M chromium(III)
nitrate.
(a) How many moles of solute are contained in 90.00 mL
of solution?
(b) How many grams of solute are contained in 90.00 mL of
solution?
(c) What volume (in milliliters) of solution is needed to obtain
0.0248 moles of solute?
Solution. Remember the definition of molarity (M): moles of
solute in one liter of solution. Cr(NO3)3 is
the solute. Thus,
moles Cr(NO3)3 =
(molarity)(volume of solution)
(a) moles Cr(NO3)3 = (0.2009 mol
Cr(NO3)3 /L)(0.09000 L)
= 0.01808 moles Cr(NO3)3
(b) mass Cr(NO3)3 = (238.01 g
Cr(NO3)3 / mol)( 0.01808 mol)
= 4.303 g
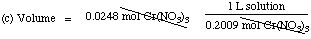
= 0.1234 L = 123 mL
3. Hint. See Example 5.10 on pp. 218-9, Kotz and Treichel.
Also the Problem-Solving Tips and Ideas on p. 238.
4. Worked Example. Suppose you need 0.500 L of 1.50 M
NH3, but only the concentrated base (14.8 M) is available.
What volume of the concentrated base (in mL) must you dilute to 0.500
L to obtain 1.50 M NH3?
Solution. You know how many moles of ammonia
you need in the final solution (0.500 L x 1.50 mol/L), so you have to
add this many moles of ammonia as the concentrated base.
moles NH3 needed = 0.500 L x 1.50 mol/L =
0.750 mol NH3
You also know that one liter of the concentrated ammonia provides
14.8 mol NH3. Thus
(0.750 mol NH3) x (1 L concentrated base /
14.8 mol) x (1000 mL / L)
= 50.7 mL concentrated NH3 is needed for dilution.
5. Worked Example. Suppose that 0.500 L of 3.00 M
NH3 is mixed with 0.750 L of 1.00 M NH3.
Assuming the total volume of the solution after mixing is 1.250 L,
what is the concentration (molarity) of NH3 in the
resulting solution?
Solution. Find the moles of
NH3 in each of the starting solutions. Add them to get
total moles, and divide by the final volume for molarity.
total moles NH3 = (0.500 L) (3.00 mol NH3/L)
+ (0.750 L) (1.00 mol NH3/L)
= 2.25 mol NH3
molarity of the final solution = 2.25 mol NH3 / 1.25 L
= 1.80 M
6. Worked Example. What volumes of 1.50 M NH3
and 6.00 M NH3 must be mixed to make 0.500 L of 2.50 M
NH3?
Solution. The solution to this problem is the
reverse of the one above. We have two unknowns, the volumes of the
two starting solutions. However, we know that the final volume (0.500
L) is the sum of the two initial volumes:
0.500 L = volume of 1.50 M NH3 + volume of
6.00 M NH3 (equation 1)
We also know that the total number of moles in the final solution
is equal to the sum of the moles from the two initial solutions:
(0.500 L)(2.50 mol/L) = total moles = 1.25 moles
NH3
= moles from 1.50 M NH3 + moles from 6.00 M
NH3 (equation 2)
Let x be the volume of 1.50 M NH3. Then from equation
1,
0.500 L = x + volume of 6.00 M NH3, or
volume of 6.00 M NH3 = 0.500 - x
Now use equation 2:
1.25 moles NH3 = (x) (1.50 mol
NH3/L) + (0.500 - x)(6.00 mol NH3/L)
Solve for x to get the answers:
x = 0.389 L of 1.50 M NH3
0.500 - x = 0.111 L of 6.00 M NH3
7. Hints. The following steps are necessary to solve this
problem.
(a) Make sure your equation is balanced.
(b) Determine the moles of one reactant from its volume and
molarity.
(c) Use the balanced equation to determine how many moles of the
second reactant are needed to react with the moles of the first
reactant (found in part b).
(d) Remember to convert moles of the second reactant to grams.
8. Hints and worked example
Hints for parts (a) and (b). Parts (a) and (b)
are like parts (a) and (b) of Question 4, Assignment 4, once you
determine moles of the two reactants. [To remind yourself about
limiting reagent problems, click
here to go to Assignment 4.]
Worked example for part (c) Suppose you mix 25.0 mL of
0.1500 M Ba(NO3)2 with 15.0 mL of 0.1000 M
Fe2(SO4)3 . What is the molarity of
the excess reactant remaining in solution after the maximum mass of
BaSO4 has
precipitated? The balanced equation is
3 Ba(NO3)2 +
Fe2(SO4)3 Æ 3
BaSO4
+ 2 Fe(NO3)3
Solution: Step 1: First determine the reactant that is in excess [you will
have done this already in order to do part (a)]
moles
Ba(NO3)2 = (0.0250 L) x (0.1500 mol / L)
= 0.00375 moles
moles Fe2(SO4)3 = (0.0150 L) x
(0.1000 mol / L) = 0.00150 moles
moles Ba(NO3)2 needed to react with 0.00150
moles Fe2(SO4)3:
0.00150 mol Fe2(SO4)3
x (3 mol Ba(NO3)2/mol
Fe2(SO4)3)
= 0.00450 mol Ba(NO3)2 needed
We don't have this much Ba(NO3)2 so
Ba(NO3)2 is limiting and
Fe2(SO4)3 is in excess
Step 2: Determine how much
Fe2(SO4)3 reacts with the
Ba(NO3)2:
0.00375 mol Ba(NO3)2 x (1 mol
Fe2(SO4)3/ 3 mol
Ba(NO3)2)
= 0.00125 mol
Fe2(SO4)3 reacts
Step 3: Determine the molarity of the excess
Fe2(SO4)3.
Amount of Fe2(SO4)3
remaining = amount to start - amount reacted
= 0.00150 mol - 0.00125 mol = 0.00025 mol (2 s.f.
now!)
Final volume of solution = 15.0 mL + 25.0 mL = 40.0 mL = 0.0400 L
Therefore the molarity of the
Fe2(SO4)3 after the reaction is:
0.00025 mol Fe2(SO4)3
/ 0.0400 L = 0.0063 M (2 s.f.)
9. Hints.
Step 1. Find the moles of one of the reactants fron
the known volume and molarity. Remember, you can't find the moles of
the other reactant directly because you only know the mass of the
entire sample, not the mass of the specific compound.
Step 2. Use the equation (stoichiometric ratio) to find moles of
the other reactant.
Step 3. Calculate the mass of the other reactant now that you know
the moles
Step 4. Determine its percentage in the original sample.
10. Hints. Determine the moles of sodium hydroxide from the
volume and molarity, and use the balanced equation to get the moles
of the unknown acid. Remember that the molar mass is the mass in
grams divided by the number of moles.
11. Worked Example. A sample of ferrous ammonium sulfate
hexahydrate
(Fe(NH4)2(SO4)2.6H2O, FAS) weighed 0.6154 g. It
required 29.35 mL of a potassium permanganate solution for titration.
What is the molarity of the KMnO4 solution? The net ionic
equation for the reaction is:
5 Fe2+ +
MnO4- + 8
H+
Æ 5 Fe3+ + Mn2+ + 4 H2O
The molar mass of
Fe(NH4)2(SO4)2.6H2O is 392.142 g/mol.
Analysis of the problem. You can calculate the
moles of ferrous ammonium sulfate hexahydrate since you know its
mass. From this and the balanced equation you can determine the moles
of permanganate, and from the moles and volume of the potassium
permanganate you can get the molarity.
Solution.
moles FAS = (0.6154 g) x (1 mol / 392.142 g)
= 0.001569329 mol
moles Fe2+ = moles FAS x (1 mol Fe2+ / mol
FAS)
= 0.001569329 mol
moles MnO4- = 0.001569329 mol
Fe2+ x (1 mol MnO4- / 5 mol
Fe2+ )
= 0.000313866 mol
moles KMnO4 = 0.000313866 mol
MnO4- x (1 mol KMnO4 / mol
MnO4- )
= 0.000313866 mol
molarity of KMnO4 = moles KMnO4 / volume of
KMnO4 (in L)
= 0.000313866 mol / 0.02935 L
= 0.010693898 M
= 0.01069 M (after rounding)
Note: for those adept at using dimensional analysis, this
question can be worked in a single step:
molarity of KMnO4 =
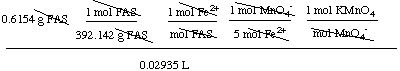
= 0.01069 M (after rounding)
12. Worked Example. A sample weighing 0.9513 g contains an
unknown amount of
Fe(NH4)2(SO4)2.6H2O. The sample required 38.75 mL
of 0.01069 M KMnO4 for titration.
(a) What is the percentage of
Fe(NH4)2(SO4)2.6H2O in the sample?
(b) What is the percentage of Fe in the sample?
Analysis of the problem. Now we know the molarity and
volume of potassium permanganate, but we do not know how much FAS we
had to begin with. This can be calculated by a series of steps that
are the inverse of Question 12.
The molar mass and balanced equation are given in Question 12.
Solution, part (a).
moles MnO4- = moles
KMnO4 (1 mol MnO4- / 1 mol
KMnO4 )
= 0.03875 L x (0.01069 mol KMnO4 / L)
= 0.00041424 mol MnO4-
moles Fe2+ = moles MnO4- x (5 mol
Fe2+ / mol MnO4- )
= 0.00041424 mol MnO4- x (5 mol
Fe2+ / mol MnO4- )
= 0.0020712 mol Fe2+
And since each mole of
Fe(NH4)2(SO4)2.6H2O (FAS) contains one mole of
Fe2+,
moles FAS = moles Fe2+
mass FAS = 0.0020712 mol FAS x 392.142 g/mol
= 0.8121996 g
% FAS = 100 * 0.8121996 g FAS / 0.9513 g sample
= 85.38 % FAS
Solution, part (b).
% Fe = 100 * mass Fe / mass sample
You know that one mole (392.142 g) of FAS contains one mole
(55.847 g) of iron. Therefore,
mass Fe = mass FAS x (55.847 g Fe / 392.142 g FAS) = 0.11567 g Fe
Therefore % Fe = 100 x 0.11567 g Fe / 0.9513 g sample
= 12.16 % Fe
13. Worked Example. Sodium thiosulfate,
Na2S2O3, is an important reagent for
titrations. Its solutions can be standardized by titrating the iodine
released when a weighed amount of potassium hydrogen iodate,
KH(IO3)2 (389.912 g/mol), is allowed to react
with potassium iodide in acidic solutions. The net ionic equations
are:
production of iodine from
KH(IO3)2: IO3- + 5
I- + 6 H+ Æ
3 I2 + 6 H2O
titration of iodine: I2 + 2
S2O32- Æ
2 I- + S4O62-
What is the molarity of a sodium thiosulfate
solution if 27.62 mL are required to titrate the iodine released from
0.1695 g of KH(IO3)2?
Analysis of the Question. There are 3 important
differences between this question and Question 12.
1. The reaction occurs in two different stages.
2. The product of the first reaction (I2) is titrated
in the second
3. There are TWO moles of iodate in one mole of
KH(IO3)2
Thus, we have to connect what we begin with
[KH(IO3)2] to the amount of iodine produced in
the first step. Then we can calculate the moles of thiosulfate that
reacted, and from that obtain the molarity of
Na2S2O3.
Solution. First find the moles of iodate:
moles IO3- =
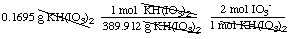
= 0.00086943 mol IO3-
Now find the moles of I2 produced from this
amount of iodate:
moles I2 produced =
0.00086943 mol IO3- x
(3 mol I2 / 1 mol IO3- )
= 0.0026083 mol I2
Next use the second equation to determine the amount of
thiosulfate needed for titration:
moles S2O32- = 0. 0023082 mol I2 x (2 mol S2O32-
/ mol I2 )
= 0.0052166 mol
S2O32-
= 0.0052166 mol Na2S2O3 (1 mol
S2O32- in each mol of
Na2S2O3 )
Molarity of Na2S2O3 = moles
Na2S2O3 / volume used for titration
= 0.0052166 mol / 0.02762 L
= 0.1889 M Na2S2O3
Back
to Chem 1P80 Home Page
Back
to Assignment Schedule
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM_1P80_Assign_6.html
Last revised: October 23 2000 by M. F. Richardson
© Brock University, 1999