1. (15 marks)
(a) Predict the product(s) for the following reactions and balance the equations:
(b) Write the balanced net ionic equation for the reaction (iii) in part 1(a) above.(i) Al + O2 Æ(ii) N2O5 + H2O Æ
(iii) CrCl3 + NaOH Æ
(c) Balance the following equation:
2. (10 marks) Fill in the blanks in the following questions:C2H5OH + O2 ÆCO2+ H2O
(a) A mole of sulfur atoms contains atoms.
A mole of S8 molecules contains molecules.(b) Consider the ionA mole of S8 molecules contains atoms.
A mole of sulfur atoms has a mass of grams.
A mole of S8 molecules has a mass of grams.
The number of protons in this ion is .(c) Which of the following elements is most likely to have an ion with a +2 charge? K, Ca, As, Se, BrThe number of neutrons is .
The number of elctrons is .
The mass number is
3. (10 marks) Give acceptable names for the following compounds
(a) AlI3
(b) CuClO2
(c) KHSO4
(d) Fe2(CO3)3
(e) N2O3
4. (10 marks) Give formulas for the following compounds
(a) strontium chloride
(b) mercury(II) bromide
(c) copper(II) oxide
(d) chromium(III) carbonate
(e) tetraphosphorus heptasulfide
5. (15 marks)
(a) (3 marks) The empirical formula of a compound is C2H4O,
and its molar mass is 264.3 g/mol. What is the molecular formula?
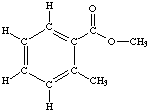
(b) (4 marks) Calculate the wt. % H in the compound whose structure is shown below:

(c) (3 marks) The density of liquid mercury is 13.6 g/cm3. What is the density in lb/ft3? Conversions:
1 lb = 454 g; 1 ft = 30.48 cm.
(d) (5 marks) The mass of 0.350 mol of a compound with a formula MBr3 is 103.4 g.
6 (10 marks) A compound contains 38.86% C, 3.80 % H, and 57.34% Cl. Calculate its empirical formula.(i) What is the molar mass of MBr3?(ii) What is M?
7. (15 marks)
From a graphical analysis of mass and volume data for Element A and Element B, answer the following questions.
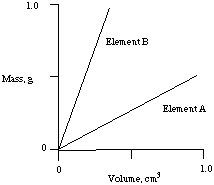
a) Which element is more dense? Explain.
b) Would element A float or sink in liquid xylene which has a density
of 0.88 g/cc? Explain
c) Of the four elements listed with their densities, select one as element A and one as Element B. Explain your choices.
Na 0.971 g/ccLi 0.534 g/cc
Al 2.70 g/cc
Zn 7.12 g/cc
8. (15 marks)
(a) (5 marks) About 200 years ago John Dalton published a theory on the composition of matter which has served as a foundation for modern chemistry. Is his postulate below still valid? If so, explain. If not, explain how the statement should be revised.
"Atoms of a given element are identical in mass and properties."
(b) (10 marks) Answer the following questions using the figures shown here.
NOTE: Each question may have more than one answer; each figure may be used more than once.

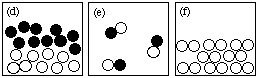
(i) Which drawing represents submicroscopic particles in a sample of solid?(ii) Which drawing represents submicroscopic particles in a sample of an element?
(iii) Which drawing represents submicroscopic particles in a sample of a compound?
(iv) Which drawing represents submicroscopic particles in a heterogeneous mixture?