Instructions:
Write all answers on this examination paper.
A periodic table is provided.Nonprogrammable calculators are allowed (no organizers!)
No other aids are allowed.
SHOW ALL WORK! GIVE UNITS!
1. (27 marks) Short Answer Questions
(a) (3 marks) Round the following calculations to the proper number of significant figures:
(i) 37.67 + 1311.3 + 7.0079 = 1355.9779(b) (3 marks) Write the formula of the compound formed between:(ii) (166.449 x 123.8) / 46.231 = 445.726595
(iii) (9.28 + 198.762) / 4.3318 = 48.02668637
potassium and sulfur(c) (4 marks) Write formulas for the following compounds:aluminum and oxygen
calcium and chlorine
dinitrogen trioxide(d) (5 marks) Fill in the blanks with the correct answer.ammonium nitrate
manganese(II) carbonate
sodium hydrogen sulfite
A mole of phosphorus atoms contains _____________ atoms.(e) (6 marks) Consider the ionA mole of P 4 molecules contains _____________ molecules.
A mole of P 4 molecules contains _____________ atoms.
A mole of phosphorus atoms has a mass of _____________ grams.
A mole of P4 molecules has a mass of _____________ grams.
The number of neutrons in this ion is _____________(f) (6 marks) Balance the following equations:The number of protons in this ion is _____________ .
The mass number is _____________ .
The number of electrons is _____________ .
The atomic weight of element X is _____________ .
The symbol for element X is _____________ .
Fe + Cl2 ---> FeCl32. (18 marks) Short CalculationsC6H6O + O2 ---> CO2 + H2O
FeS + O2 ---> Fe2O3 + SO2
(a) (5 marks) You are demonstrating the CHEM 1P80 class and you need to prepare 12.00 L of a 25.00% sugar solution. Its density is 1.1036 g/mL. What mass (in grams) of sugar would you take to make up the solution?
(b) (4 marks) Water has a density of 1.00 g/cm3. What is its density in the following units?
kg/L(c) (3 marks) What mass of Fe2(SO4)3 (399.88 g/mol) must be taken in order to obtain 0.200 moles of iron?g/mm3
(d) (6 marks)
(i) Calculate the formula weight of Cu(NO3)2.3H2O.3. (10 marks) A sample was analyzed for the percentage BaCl2. The sample was dissolved in water, excess AgNO3 was added to precipitate AgCl, and the AgCl was filtered and weighed. If 0.4400 g of the sample yielded 0.2348 g of AgCl, what is the % BaCl2 in the sample? The balanced equation is(ii) What is the percentage of oxygen in Cu(NO3)2.3H2O ?
BaCl2 (aq) + 2 AgNO3 (aq) ---> 2 AgCl (s) + Ba(NO3)2 (aq)
Molar masses: BaCl2 208.23 g/mol; AgCl 143.32 g/mol
4. (12 marks) Sulfur reacts with fluorine to produce sulfur tetrafluoride according to the following equation:
S8 + 16 F2 ---> 8 SF4
(a) How many grams of fluorine are required to react with 0.350 moles of sulfur?(b) What mass of sulfur tetrafluoride can be produced?
(c) If 143 g of SF4 are actually obtained, what is the percent yield of SF4?
5. (15 marks) (a) Briefly explain how combustion analysis is carried out on an organic compound. Include a sketch of the apparatus.
(b) A 0.6825 sample of a compound containing C, H, and O gave 1.333 g CO2 and 0.682 g H2O upon combustion analysis. What is the empirical formula of the compound?
Molar masses: CO2 44.01 g/mol; H2O 18.02 g/mol
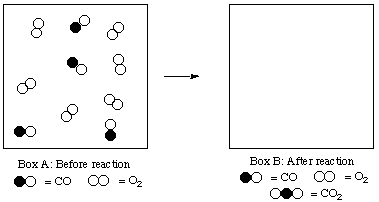
6. (6 marks) Gaseous carbon monoxide (CO) and O2 react to form CO2:
2 CO + O2 ---> 2 CO2
A reaction mixture is represented in Box A below.
(a) Show the situation that will exist after reaction has occurred in Box B below.(b) How many molecules of CO2 can be formed from these reactants?
(c) Which reactant is in excess? By how many molecules?

7. (12 marks; 2 marks each) Choose the best answer to each of the following questions:
(i) If 4.00 g of element A react completely with 8.00 g of element B, which of the following statements must be true?
a) The maximum mass of product that can be formed is 12.00 g.(ii) In the Millikan oil drop experiment, the charge on oil droplets was observed by their behavior between a positively charged plate and a negatively charged plate. The fundamental charge on an electron was determined to be -1.60 x 10-19 coulombs by observing thatb) The reaction requires 4 moles of A to react with 8 moles of B.
c) The maximum amount of product that can be formed is 12.00 moles.
d) The atomic weight of B is double the atomic weight of A.
e) The density of B is greater than the density of A.
a) The charge on all the droplets was +1.60 x 10-19 coulombs.(iii) The number of isotopes in a sample of a pure element can best be determined experimentally with theb) The charge on all the droplets was -1.60 x 10-19 coulombs.
c) The charge on all the droplets was a multiple of -1.60 x 10-19 coulombs.
d) The charge on all the droplets was -1.60 x 1019 coulombs.
e) The charge on all the droplets was +1.60 x 1019 coulombs.
a) electroscope.(iv) Which group of compounds are ALL ionic?b) mass spectrometer.
c) electron microscope.
d) cathode ray tube.
e) scanning tunneling microscope.
a) H2O, NaCl, CS2(v) Which of the three statements regarding compounds is (are) true:b) NaCl, CH4, CaCl2
c) H2O, FeCl3, CO2
d) CaCl2, FeCl3, NaCl
e) CaCl2, FeCl3, CO2
1. A compound has different properties than the elements of which it is a compound.
2. A compound has a definite percentage composition by mass of its combining elements.
3. A compound must be composed of molecules.
a) 1, 2, and 3(vi) Which of the following sets of compounds illustrate the law of multiple proportions as set forth by John Dalton?b) 1 only
c) 2 only
d) 3 only
e) 1 and 2 only
a) CO2, H2O, N2H4, C2H4b) CO2, SO2, SiO2, TiO2
c) NO, NO2, N2O, N2O5
d) NO2, NH3, H2NOH, H2O
d) C2H6, H6C2, CH3CH3, (CH3)2
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/Midterm_Exam_1_1998.html
Created September 27, 2000 by M. F. Richardson
© Brock University, 2000