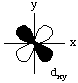 |
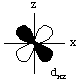 |
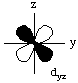 |
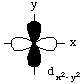 |
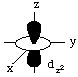 |
A. Electrons in d-Orbitals
B. Splitting of the d-Orbitals in an Octahedral Field
C. Consequences of d-Orbital Splitting: Magnetism
D. Consequences of d-Orbital Splitting: Colour
A. Electrons in d-Orbitals
All d-orbitals have the same energy (in spite of their different shapes and/or orientations) on a bare metal ion. However, some d-orbitals have different energies from the others in a metal complex. This is called d-orbital splitting.Consider the 5 d-orbitals in an xyz coordinate system. We will not try to give perspective drawings, rather we will rotate the coordinate system so that it is easy to draw the orbitals.
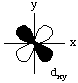 |
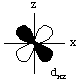 |
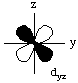 |
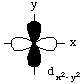 |
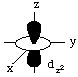 |
The odd-shaped dz2 orbital results because there are six solutions to the Schroedinger equation for the angular momentum quantum number l (the d-orbitals), but only 5 solutions are independent. The combination of two orbitals produces the unique dz2 orbital:
B. Splitting of the d-Orbitals in an Octahedral Field
Let's look at what happens to the energies of electrons in the d-orbitals as six ligands approach the bare metal ion:C. Consequences of d-Orbital Splitting: Magnetism
If we compare the dxy and the dx2-y2, we can see that there is a significant difference in the repulsion energy as ligand lone pairs approach d-orbitals containing electrons.
Electrons in the dxy orbital are concentrated in the space between the incoming ligands. Electrons in the dx2-y2 orbital point straight at the incoming ligands. Now, dxz and dyz behave the same as dxy in an octahedral field, and dz2 behaves the same as dx2-y2. This means that the d-orbitals divide into two groups, one lower energy than the other, as shown in the following diagram.
The dxy, dxz, and dyz orbitals are collectively called the t2g orbitals, whereas the dz2 and dx2-y2 orbitals are called the eg orbitals. The octahedral splitting energy is the energy difference between the t2g and eg orbitals. In an octahedral field, the t2g orbitals are stabilized by 2/5 Deltao, and the eg orbitals are destabilized by 3/5 Deltao.
Let's consider the complexes [Fe(H2O)6]Cl3 (mu = 5.9 B.M.; 5 unpaired electrons) and K3[Fe(CN)6] (mu = 1.7 B.M.; 1 unpaired electron).The free Fe3+ ion is a d5 ion. The two complexes are 6-coordinate and octahedral. First let's look at the d-orbital diagram for [Fe(H2O)6]3+:
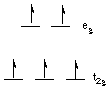
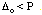
d-orbital diagram for [Fe(H2O)6]3+
The first three electrons go into t2g orbitals unpaired. The 4th and 5th electrons must choose whether to pair up with electrons already in t2g (which costs energy) or to go into higher energy eg orbitals (which also costs energy). In this case, the splitting energy is less than the pairing energy so the 4th and 5th electrons go into the eg orbitals. Now let's consider the [Fe(CN)6]3- ion. Again we have five d-electrons. However, there is only one unpaired electron, so the 4th and 5th electrons must pair with electrons already in t2g orbitals. this happens because the octahedral splitting energy is much greater in the hexacyanoferrate(III) ion than it is in the hexaaquoiron(III) ion. That is, the cyanide ligand causes a much greater d-orbital splitting than water does.
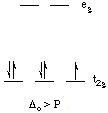
d-orbital diagram for [Fe(CN)6]3-
The first three electrons go into t2g orbitals as before. Now, however, the splitting energy is much greater so it is less energetically costly for electrons to pair up in the t2g orbitals than to go into the eg orbitals. [Fe(CN)6]3- is called a low-spin complex because it has the lowest number of unpaired spins (electrons) possible for an octahedral iron(III) complex. [Fe(H2O)6]3+ is called a high-spin complex because it has the highest number of unpaired spins for an octahedral iron(III) complex. The terms "high-spin" and "low-spin" do not refer to specific numbers of unpaired electrons, but rather to different electron configurations in d-orbital diagrams that result from the pairing energy being greater than or less than the splitting energy.
D. Consequences of d-Orbital Splitting: Colour
Colour in transition metal complexes is due to an electron being excited from one d-orbital to a higher-energy d-orbital. In the case of octahedral complexes, an electron is moves from a t2g orbital to an eg orbital. The energy difference for the first transition series generally falls in the visible region. Absorption of one colour in the visible spectrum results in the ion having the complementary colour.The amount of d-orbital splitting depends on the ligands. thus different ligands have different splitting energies, and different colours result.
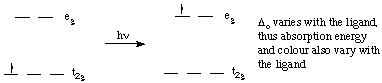
Excitation of an electron from a t2g orbital to an eg orbital causes the red colour of [Ti(H2O)6]3+ ions. (Ti3+ is a d1 ion).
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM2P32_Lecture_9.html
Created January 23, 2001 by M. F. Richardson
© Brock University, 2001