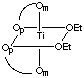
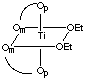
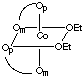

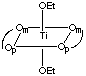
There are 5 geometric isomers with this formula. Om designates the oxygen closest to the methyl group, Op the oxygen closest to the phenyl group.
 |
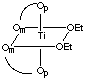 |
 |
|
|
|
|
 |
 |
|
|
|
|
The reason that isomers are so important in drug design is that normally,
only one particular isomer is effective in treating the condition. Other
isomers are less effective or even harmful! The classic example is thalidomide,
where one enantiomer helped women overcome morning sickness associated
with the early weeks of pregnancy, and the opposite enantiomer caused the
horrendous birth defects (lack of arms and legs) seen in what are now known
as the "thalidomide babies."
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/L7_SelfTest1_budotitane.html
Created January 18, 2001 by M. F. Richardson
© Brock University, 2001