Lecture 7
Stereochemistry in Octahedral, Tetrahedral, and
Square Planar Geometries
A. Geometric Isomerism in Octahedral Complexes
B. Optical Isomerism in Octahedral Complexes
C. Isomerism in Tetrahedral and Square Planar
Complexes
A. Geometric Isomerism for Some Common
Octahedral Complex Compositions
1. Composition MA4B2 has 2 geometric
isomers, cis and trans. We have already seen in Lecture 6 that there
are two geometric isomers possible for this composition when the coordination
geometry is octahedral: cis and trans. The key is to not get confused by
different ways of drawing these isomers. You should especially remember
that all six positions on an octahedron are equivalent: there are not axial
and equatorial positions as there are for a trigonal bipyramid.
For example, both drawings below represent the cis-tetraamminedichloroplatinum(IV)
ion, in which the Cl-Pt-Cl bond angle is 90o:
4Cl2.gif)
And both of these drawings represent the trans-tetraamminedichloroplatinum(IV)
ion, in which the Cl-Pt-Cl bond angle is 180o.
4Cl2.gif)
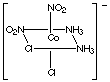
2. Composition MA3B3 has two geometric isomers,
fac and mer.The fac- isomer (short for "facial") gets its name because
all three chlorides are coordinated on one face of the octahedron. The
mer-isomer (short for "meridional") has the three chloride ions coordinated
in a plane that includes the metal ion. (This corresponds to the meridian
on a sphere, which is any plane through the sphere that contains the center.)
3Cl3.gif)
|
3Cl3.gif)
|
fac-triamminetrichlorocobalt(III)
All three Cl-Co-Cl bond angles are 90o.
|
mer-triamminetrichlorocobalt(III)
Two Cl-Co-Cl bond angles are 90o, the other is 180o.
|
3. Composition MA2B2C2
has five geometric isomers. It's just a matter of working out all the
cis and trans possibilities for each pair of ligands. Consider the cobalt
complex [Co(NH3)2Cl2(NO2)2]-.
2CoCl2(NH3)2.gif)
|
2CoCl2(NO2)2.gif)
|
|
cis-diammine-cis-dichloro-trans-di-N-nitritocobaltate(III) ion
|
trans-diammine-cis-dichloro-cis-di-N-nitritocobaltate(III) ion
|
2(NO2)2.gif)
|
2(NO2)2.gif)
|
|
cis-diammine-trans-dichloro-cis-di-N-nitritocobaltate(III) ion
|
cis-diammine-cis-dichloro-cis-di-N-nitritocobaltate(III) ion
|
2(NO2)2.gif)
|
|
|
trans-diammine-trans-dichloro-trans-di-N-nitritocobaltate(III)
ion
|
|
4. Composition M(A-B)3 has two geometric isomers, fac
and mer. Here, A-B represents an unsymmetrical bidentate chelating
agent such as the aminoethanethiolate ion, NH2CH2CH2S-,
or the benzoylacetonate ion whose resonance forms are shown below. The
donor atoms are different for aminoethanethiolate so it is clearly unsymmetrical.
However, even though the donor atoms are same (both oxygens) for benzoylacetonate,
the two "ends" of the chelating anion are different: one oxygen is close
to a methyl group, the other to a phenyl group
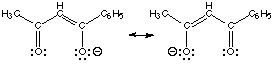
resonance structures forbenzoylacetonate
3.gif)
|
3.gif)
|
|
fac-isomer, Co(bzac)3. Op is the oxygen closest
to the phenyl group, Om is the oxygen closest to the methyl
group.
|
mer-isomer, Co(bzac)3 . Here the Om's are
all meridional, as are the Op's
|
5. Composition M(A-A)2B2 has two geometric
isomers, cis and trans. Here, A-A represents a symmetrical bidentate
ligand such as acetylacetonate, oxalate, or ethylenediamine. The isomers
for [Co(en)2Cl2]+ are shown below.
2Cl2.gif)
|
2Cl2.gif)
|
|
cis-dichlorobis(ethylenediamine)cobalt(III) ion
|
trans-dichlorobis(ethylenediamine)cobalt(III) ion
|
6. M(trien)B2 has 3 geometric isomers. Trien is a
linear tetradentate ligand,
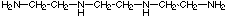
The flexible trien ligand can coordinate to an octahedral center in
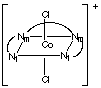
3 different ways as shown below for [Cr(trien)Cl2]+.
Naming the complexes requires a method beyond the scope of CHEM 2P32. Nm
and Nt designate the middle NH groups and the terminal NH2
groups, respectively, on the ligand.
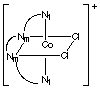
|
|
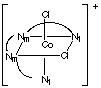
|
|
Isomer A: both chlorides are trans to the middle nitrogens
|
Isomer B: the chlorides are trans to each other
|
Isomer C: one chloride is trans to a middle nitrogen, the other
is trans to a terminal nitrogen.
|
SELF-TEST 1: GEOMETRIC ISOMERS. Budotitane is a 6-coordinate
octahedral titanium complex that has been developed for treating colon
cancer. Its formula is [Ti(bzac)2(OEt)2], where bzac
is the benzoylacetonate ligand shown above.
The OEt ligand is ethoxide, CH3CH2O-.
Draw the possible geometric isomers.
Answer.
SELF-TEST 2: GEOMETRIC ISOMERS. Draw the geometric isomers of
the octahedral complex, [Co(dien)Br2Cl]. dien is the linear
tridentate ligand NH2CH2CH2NHCH2CH2NH2.
Answer.
B. Optical Isomerism for Some Common Octahedral
Complex Compositions
1. M(A-A)3 has optical isomers. See the previous
lecture (click
here).
This means that any octahedral tris chelate, whether with a symmetrical
or an unsymmetrical bidentate ligand, is chiral and will have optical isomers.
2. cis-M(A-A)2X2 has optical isomers. It
follows that cis-M(A-B)2X2 is chiral and will also
has optical isomers. The optical isomers of [Co(en)2Cl2]+
are shown below.
2Cl2optical.gif)
3. The cis-cis-cis geometric isomer of MA2B2C2
is chiral. The cis-diammine-cis-dichloro-cis-di-N-nitritocobaltate(III)
ion, one of the 5 geometric isomers of [Co(NH3)2Cl2(NO2)2]-
shown above, has no plane of symmetry in
the ion and thus has optical isomers.
4. All 15 geometric isomers of the complex MABCDEF are chiral.
It's unlikely that all 15 isomers with this composition would ever be synthesized!
But any that are will be chiral and have optical isomers.
C. Geometric and Optical Isomerism for
Some Tetrahedral and Square Planar Complexes
In the following examples we will contrast the number and types
of isomers for tetrahedral vs. square planar complexes with a particular
composition. The different number and kinds of isomers obtained for a given
composition allowed a determination of geometry in the days before x-ray
crystallography and other modern techniques were available to researchers.
1. Tetrahedral MA2B2 has no isomers, but square
planar MA2B2 has geometric isomers. Four-coordinate
platinum(II) complexes are always square planar, whereas 4-coordinate cobalt(II)
complexes are tetrahedral. The cis and trans isomers of diamminedichloroplatinum(II)
are shown below.
2Cl2.gif)
On the other hand, tetrahedral diamminedichlorocobalt(II) has no isomers,
either geometric or optical. (As you would expect, given your familiarity
with such carbon compounds as CH2Cl2).
2Cl2.gif)
2. Tetrahedral MABCD has optical isomers but not geometric isomers;
square planar MABCD has geometric isomers but not optical isomers. In
the sketch below, M can be any metal that exhibits square planar coordination.
The three isomers place A trans to C, A trans to B, and A trans to D. None
of these is chiral (the square plane is the plane of symmetry in these
complexes).
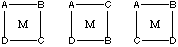
Tetrahedral MABCD is analogous to carbon bonded to four different groups,
and thus has optical isomers but not geometric isomers.
3. Neither tetrahedral M(A-A)2 nor square planar M(A-A)2
has isomers, as long as the chelating ligands are symmetrical and there
are no chiral centers on the ligands.
4. Tetrahedral M(A-B)2 has optical isomers but not geometric
isomers; square planar M(A-B)2 has geometric isomers but not
optical isomers. Real examples of unsymmetrical A-B ligands are aminoethanethiolate
and benzoylacetonate complexes. Aminoethanethiolate is NH2CH2CH2S-;
see
above for the benzoylacetonate structure.
Consider the aminoethanethiolate complexes of square planar platinum(II)
and tetrahedral cobalt(II).
2geom.gif)
|
2optical.gif)
|
|
geometric isomers of [Pt(NH2CH2CH2S)2]
|
optical isomers of [Co(NH2CH2CH2S)2]
|
Back
to Lecture Schedule
Back
to CHEM 2P32 Home Page
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM2P32_Lecture_7.html
Created January 18, 2001 by M. F. Richardson
© Brock University, 2001
4Cl2.gif)
4Cl2.gif)
3Cl3.gif)
3Cl3.gif)
2CoCl2(NH3)2.gif)
2CoCl2(NO2)2.gif)
2(NO2)2.gif)
2(NO2)2.gif)
2(NO2)2.gif)
3.gif)
3.gif)
2Cl2.gif)
2Cl2.gif)
![]()
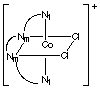

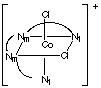
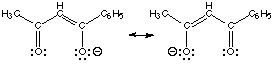
2Cl2optical.gif)
2Cl2.gif)
2Cl2.gif)
2geom.gif)
2optical.gif)