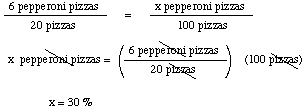Lecture 3
A. States of Matter: Summary
Matter may be heterogeneous or
homogeneous
Homogeneous matter may be a pure substance or a
solution
Pure substances may be elements or compounds
Pure solids may have network structures or molecular
structures
See
Lecture
1,
Lecture
2, and Figure 1.1 in the 4th edition of Kotz and Treichel
B. Why Pay Attention to Units?
Read "The Gimli Glider", p. 42 in the 4th edition of
Kotz and Treichel
C. Percentages
Percent: from the Latin per centum, "per
hundred"
For example, assume that the Chemistry Club orders 20 pizzas and 6
are pepperoni. Let's calculate the percentage of pepperoni pizzas,
which is the number of pizzas that would be pepperoni IF the
Chemistry Club had ordered 100 pizzas.
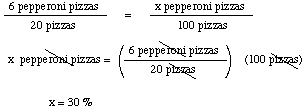
Note that 6 pepperoni pizzas/20 pizzas represents the
fraction of the pizzas that are pepperoni. Thus,
% pepperoni pizzas = (fraction of pepperoni
pizzas) * 100
Percentage is used in many contexts, e.g.,
- % right on an exam (score out of 100 possible marks = fraction
correct * 100)
- % composition (how many jellybeans in 100 are black?) =
fraction black * 100
- % error = 100*(accepted value-your value)/accepted value
- mass % of X = (grams of X/100 grams of sample) = fraction of X
by mass * 100
- volume % of X = (mL of X in 100 mL of sample) = fraction of X
by volume * 100
D. Example: Mass % and its use in calculations
A sample of iron ore contains 32.5 mass % of the iron
mineral Fe2O3. Fe2O3 is
69.9 mass % iron. How much iron ore must be processed in order to
produce 10.0 kg of metallic iron?
32.5 mass % Fe2O3 = 32.5 kg
Fe2O3/100 kg iron ore
69.9 mass % iron = 69.9 kg Fe/100 kg Fe2O3
Therefore,
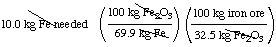
= 44.0 kg iron ore must be processed to yield 10.0 kg of metallic
iron
E. Example: Volume % and its use in calculations
U. S. beer is typically 3.8 mass % alcohol, whereas
Canadian beer is usually 5.0 volume % alcohol. Convert 3.8 mass %
alcohol to volume % alcohol. The density of beer is 1.010 g/mL; the
density of pure alcohol is 0.7893 g/mL.
3.8 mass % means that there is 3.8 g alcohol in 100 g
beer
Volume % is the volume of alcohol in 100 mL of beer
So convert the masses to volumes.
3.8 g alcohol * (1 mL alcohol/0.7893 g) = 4.81 mL alcohol
100 g beer * (1 mL beer/1.010 g beer) = 99.0 mL beer
Thus, the volume % alcohol is the volume fraction of alcohol * 100
= (4.81 mL alcohol/99.0 mL beer) * 100
= 4.86% alcohol. (Round to 4.9% since there are 2 s.f. in 3.8
mass %)
Hence, Canadian beer and American beer have about the same
alcoholic strength.
Back
to Lecture Schedule
Back
to CHEM 1P80 Home Page
This page is
http://chemiris.labs.brocku.ca/~chemweb/courses/chem180/CHEM1P80_Lecture_3.html
Last modified September 19, 2000 by M. F. Richardson
© Brock University, 2000