Geometric isomers have different spatial arrangement
results in different geometries (different bond angles or different distances
between nonbonded atoms, for example).
Organic example: cis- and trans-2-butene, CH3CH=CHCH3
Optical isomers have the same geometrical parameters but are
related as nonsuperimposable mirror images. (In other words, the
molecule or ion is chiral.). Optical isomers get their names because
they are able to rotate a plane-polarized light beam to the left or to
the right.
Organic example: CHFClBr. A carbon atom with four different groups attached
to it has a nonsuperimposable mirror image.
There are many types of structural isomers in transition metal
complexes. We will explore three of them.
1. Ionization isomers. Ligands inside the coordination sphere
exchange places with ligands outside the coordination sphere. Ionization
isomers are so-named because they give different ions when dissolved in
water.
Example 1. There are two isomers with the formula PtCl2SO4.4NH3.
Solutions of both are neutral, meaning that the ammonia must be coordinated
to the platinum(IV) ion. [Pt(NH3)4Cl2]SO4
and [Pt(NH3)4Cl(SO4)]Cl.
Isomer A does not react with silver nitrate but gives a white precipitate
of BaSO4 when a solution of barium chloride is added to it.
Thus chloride is coordinated to platinum but sulfate is not.
Isomer B does not react with barium chloride but gives a white precipitate
of AgCl when a solution of silver nitrate is added to it. Thus sulfate
is coordinated to platinum but chloride is not.
Thus Isomer A is [Pt(NH3)4Cl2]SO4
and Isomer B is [Pt(NH3)4Cl(SO4)]Cl (platinum
(IV) has a coordination number of 6, so one of the chlorides remains coordinated
to the platinum).
Example 2. There are three compounds with the formula CrCl3.6H2O.
One is violet, one is grey-green, and the third is deep green.
The violet isomer produces 3 moles of silver chloride upon reaction
with silver nitrate, and does not lose water in a desiccator.
The grey-green isomer gives 2 moles of silver chloride upon reaction
with silver nitrate, and loses one mole of water when stored in a desiccator.
The deep green isomer gives 1 mole of silver chloride upon reaction
with silver nitrate, and loses two moles of water when stored in a desiccator.
Thus the three isomers are [Cr(H2O)6]Cl3
(violet), [Cr(H2O)5Cl]Cl2.H2O
(grey-green), and [Cr(H2O)4Cl2]Cl.2H2O
(deep green).
Note that the chloride ions that react with silver nitrate are the ones
not bonded to the chromium(III) ion, and the water molecules that are lost
in a desiccator are the uncoordinated ones.
The chromium complexes in this example are sometimes said to be an example
of hydrate isomerism, a subclass of ionization isomerism in which
one or more water molecules exchange with ions.
2. Linkage isomers. Linkage isomers can exist when one or more ambidentate
ligands is bonded to a metal ion.
Example 1.
[Pt(NH3)3(NCS)]+
and [Pt(NH3)3(SCN)]+
The two isomers differ in the mode of thiocyanate bonding. In the first
isomer, thiocyanate is bonded through nitrogen; in the second it is bonded
through sulfur.
Example 2. A compound with the formula CoCl2(NO2).5NH3
has two isomers, one yellow and one red. Each precipitates two moles of
silver chloride, therefore both chloride ions are outside the cobalt(III)
coordination sphere. Neither has an aqueous solution that is basic to pH
paper, therefore all the ammonias are bonded to cobalt (III).
The obvious possibility is that the ambidentate nitrite group is differently
bonded in these two complexes: [Co(NH3)5NO2]Cl2
and [Co(NH3)5ONO]Cl2
Werner assigned structures to each based on color similarities to other
known cobalt(III) complexes. [Co(NH3)6]Cl3
is yellow and has 6 nitrogen donor atoms bonded to cobalt; [Co(NH3)5(OH2)]Cl3
is red and has 5 nitrogen donor atoms and an oxygen bonded to cobalt. Thus
the yellow isomer of CoCl2(NO2).5NH3
contains N-bonded nitrite and is [Co(NH3)5NO2]Cl2;
the red isomer contains O-bonded nitrite and is [Co(NH3)5ONO]Cl2.
Today we would assign the structures on the basis of infrared spectra:
N- and O-bonded nitrite have different N-O stretching frequencies.
3. Coordination isomers involve ligand exchange between coordination
spheres of two metal ions that are part of the same compound.
Example 1. Two compounds are known that contain two
Pt(II) ions, four ammonia molecules, and four chloride ions. They are:
[Pt(NH3)4]2+[PtCl4]2-
and [Pt(NH3)3Cl]+[Pt(NH3)Cl3]-
[Pt(NH3)2Cl2] has the same ratio of
atoms, but does not have the same overall formula; hence it is not a coordination
isomer of the above compounds.
Example 2. Three compounds are known that contain one Pt(II)
ion, one Pt(IV) ion, four ammonia molecules, and six chloride ions. They
are:
[PtII(NH3)4]2+[PtIVCl6]2-
[PtII(NH3)3Cl]+[PtIV(NH3)Cl5]-
[PtIV(NH3)4Cl2]2+
[PtIICl4]2-
TEST YOURSELF: Coordination Isomers. Are there other coordination
isomers besides these three that contain one Pt(II) ion, one Pt(IV) ion,
four ammonia molecules, and six chloride ions? If so give their condensed
strucutres as has been done above.
Answer
1. Geometric Isomers are found in square planar and
octahedral complexes. Examples for square planar coordination are the cis-
and trans-isomers of diamminedichloroplatinum(II):
2.gif)
Note the convention of drawing a square with the metal ion in the center
and the ligands at the corners of the square.
An example of geometric isomers in octahedral complexes are the cis-
and trans-isomers of the tetraamminedichlorocobalt(III) ion:
4Cl2.gif)
Note that there are several different ways to represent an octahedrally
coordinated metal ion; which way you choose depends on what you are trying
to show.

2. Optical Isomers are usually associated with tetrahedral or octahedral
geometries. The [Co(NH3)ClBrI]- ion is tetrahedral,
with four different groups bonded to the cobalt, and has two nonsuperimposable
mirror images:
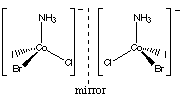
Thus this complex is exactly analogous to carbon compounds. It is also
true that an octahedral metal ion bonded to 6 different ligands would be
chiral (there are no known examples of such a complex as far as I know):
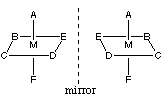
However, there are instances of chirality and optical isomerism that
do not depend on having four different groups attached to a tetrahedral
central atom. For example, thetris(ethylenediamine)cobalt(III)
ion is chiral, in spite of the fact that the three ethylenediamine ligands
are all the same and are themselves symmetrical:
3.gif)
One of the alternate ways of showing the octahedron shows the right/left
handed nature of these two isomers more clearly:
3alt.gif)
Additional examples of geometric and optical isomerism will be considered
in the next lecture.
SUMMARY SELF TEST: Sketch all of the isomers possible (including
geometric) for a compound with the formula Pt(NCS)2SO4.4NH3.
Platinum(IV) is 6-coordinate with an octahedral geometry. Assume that the
ammonia molecules are bonded to the platinum(IV) in every isomer.
Answer.
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/CHEM2P32_Lecture_6.html
Created January 18, 2001 by M. F. Richardson
© Brock University, 2001
2.gif)
4Cl2.gif)


3.gif)
3alt.gif)
